Figure 1. Neutrophil intrinsic regulators of NETosis.
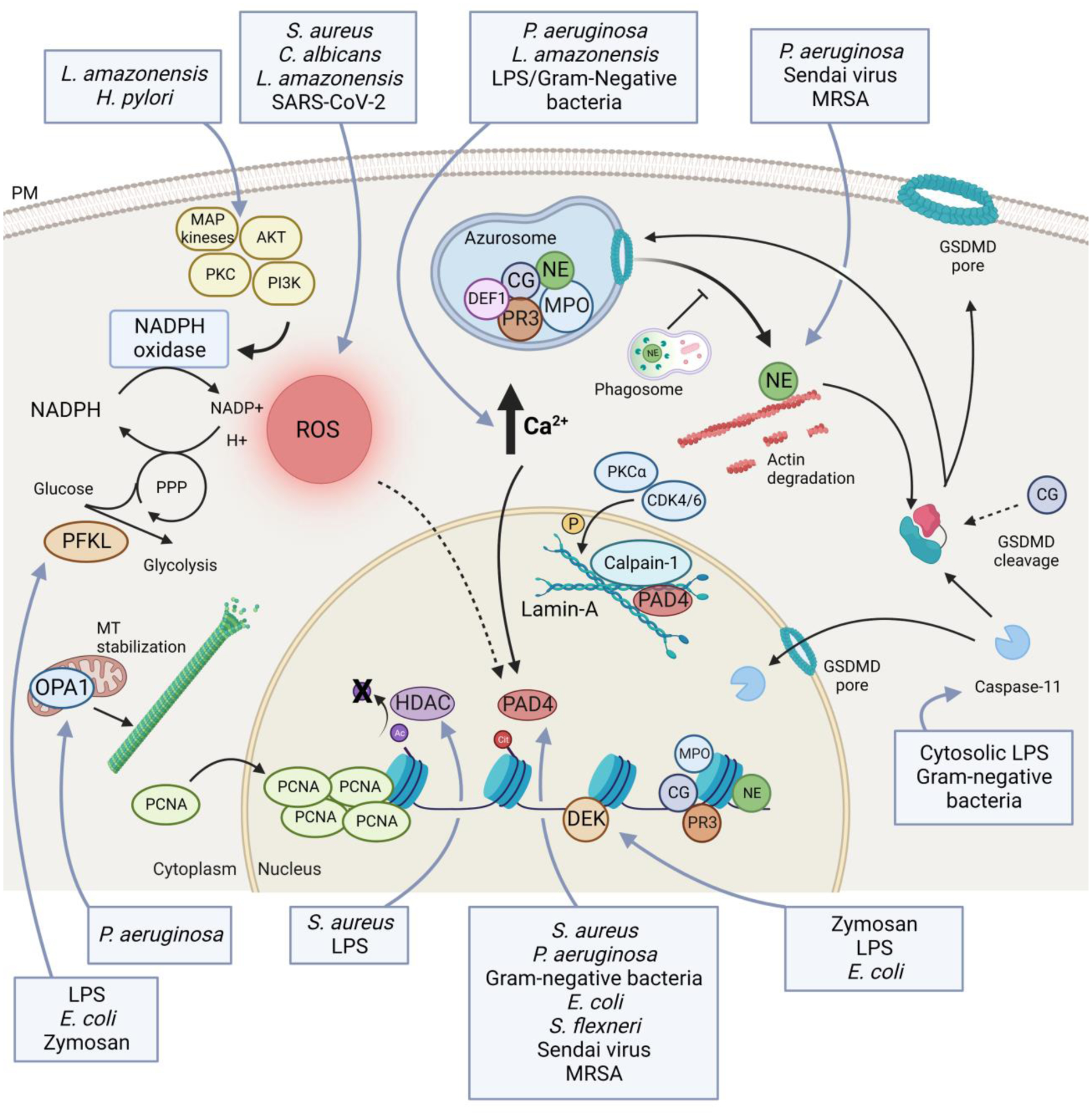
Schematic representation of the main cell-intrinsic regulators of NETosis. ROS production has an important role in regulating the release of NETs. ROS are mainly generated by the NADPH oxidase, that is regulated by several kinases, such as AKT, PI3K, PKC and MAPKs. Amount of ROS is also determined by levels of NADPH that are indirectly controlled by phosphofructokinase-1 liver type (PFKL) [70]. An increase in intracellular calcium levels is required for NETosis. Increased calcium levels, possibly in association with production of ROS, activate protein-arginine deiminase 4 (PAD4) that mediates histone citrullination and facilitates chromatin decondensation. PAD4, in association with calpain-1, PKCα and CDK4/6, mediates the disruption of the structural rigidity of the nucleus governed by the lamin-A network [51,73,74]. Histone citrullination, required to chromatin decondensation, occurs after deacetylation by class I/IIb histone deacetylase (HDAC) [72]. Other enzymes central to the formation of NETs are contained in the azurosome, and are mainly represented by myeloperoxidase (MPO), neutrophil elastase (NE), cathepsin G (CG), proteinase 3 (PR3) and defensin-1 (DEF1). Upon neutrophil activation, these enzymes translocate into the nucleus where they promote chromatin decondensation. Before moving to nuclei, NE is released in the cytoplasm and binds and degrades F-actin to arrest actin dynamics and to inhibit neutrophil migration [22]. The release of NE into the cytosol is mediated by ROS and MPO, and it is facilitated by gasdermin-D (GSDMD), but it does not require granule disruption [22,66]. GSDMD is also necessary to allow caspase-11 translocation into the nucleus, where it mediates histone clipping [40]. Moreover, GSDMD facilitates DNA release into the cytosol first, and then extracellularly by creating membranes pores. GSDMD is activated upon being cleaved by NE, caspase-11, and, potentially, by CG. Mitochondrial optic atrophy 1 (OPA1) is key to maintain ATP production and, consequently, for microtubule stability that is essential for NET release [71]. Lastly, further chromatin disassembly is accelerated by massive nuclear translocation of proliferating cell nuclear antigen (PCNA) [75] and by chromatin-binding protein DEK [59]. Blue boxes indicate pathogens and/or PAMPs that drive specific steps in the induction of NETs.
