Abstract
One of the hallmarks of vascular aging is increased pulse pressure. This elevated pulse pressure is associated with deleterious effects on cerebral vascular function; however, it is unknown if age modulates the susceptibility to high pulse pressure. To examine the effects of age on the cerebral artery response to pulse pressure, we studied isolated cerebral arteries collected from young (6.1±0.2 mo) and old (26.7±0.5 mo) male C57BL/6 mice. Isolated cerebral arteries were exposed ex vivo to static pressure, low pulse pressure (25 mmHg), and high pulse pressure (50 mmHg). In cerebral arteries from young mice, endothelium-dependent dilation was similar between the static and low pulse pressure conditions. Exposure to high pulse pressure impaired endothelium-dependent dilation in cerebral arteries from young mice, mediated by less nitric oxide bioavailability and greater oxidative stress. Cerebral arteries from old mice had impaired cerebral artery endothelium-dependent dilation at static pressure compared with young cerebral arteries. However, exposure to low or high pulse pressure did not cause any further impairments to endothelium-dependent dilation in old cerebral arteries compared with static pressure. The old cerebral arteries had less distension during exposure to high pulse pressure and greater stiffness compared with young cerebral arteries. These results indicate that acute exposure to high pulse pressure impairs endothelium-dependent dilation in young, but not old, cerebral arteries. The greater stiffness of cerebral arteries from old mice potentially protects against the negative consequences of high pulse pressure.
Keywords: Aging, cerebrovascular, pulsation, oxidative stress, arterial stiffness
1. Introduction
Vascular aging is characterized by an increased large artery stiffness that is associated with higher pulse pressure (PP)(Mitchell 2018). Higher PP, as well as higher blood flow pulsatility, are associated with neurodegenerative diseases and cognitive impairments (Qiu et al. 2003; Tsao et al. 2013; Weigand et al. 2022). Higher PP is also related to cerebral microvascular damage (Tarumi et al. 2019; Tsao et al. 2013), suggesting that high PP leads to cerebrovascular impairments. It is crucial to understand the mechanisms that underlie this relation between high PP and cerebrovascular impairment because these may provide a better understanding of the etiology of cerebrovascular and neurodegenerative diseases.
Elevated PP is known to be associated with endothelial cell dysfunction (McEniery et al. 2006) (Beigel et al. 2010; Ceravolo et al. 2003). Ex vivo exposure to high PP impairs endothelial function in cerebral arteries from young mice (Raignault et al. 2017). At the same time, old age is associated with cerebral artery endothelial cell dysfunction (Mayhan et al. 1990; Toth et al. 2014; Walker et al. 2014). However, the effect of high PP on old cerebral artery endothelial function is unknown. Advancing age is often associated with a loss of resiliency to damage (Ferrucci et al. 2020), suggesting that old arteries may be more vulnerable to the damage caused by high PP. For example, high PP also reduces the myogenic response of cerebral arteries, and this impairment occurs in arteries from only old mice, and not from young mice (Springo et al. 2015). Thus, it is important to understand the effects of high PP on endothelial function in aged cerebral arteries.
One of the hallmarks of advancing age is an increase in oxidative stress that plays a key role in attenuating arterial endothelial function (Donato et al. 2015; Taddei et al. 2001). Age-related increases in superoxide production inhibit vasodilation through the reaction with nitric oxide (NO), thereby limiting NO bioavailability (Donato et al. 2015). However, not all reactive oxygen species (ROS) have similarly deleterious effects on endothelial function. There is evidence that hydrogen peroxide mediates endothelium-dependent vasodilation in cerebral arteries during static pressure, but is more harmful during pulsatile pressure (Raignault et al. 2017). Importantly, the contributions of NO vs. hydrogen peroxide to cerebral artery vasodilation is not only modulated by PP, but also dependent on the vasodilatory stimulus (Katusic et al. 1989; Modrick et al. 2009a). Thus, the role of ROS in facilitating endothelium-dependent dilation is complex and may be affected by the interaction of PP and age.
Advancing age is also associated with changes to arterial structure. Increased PP leads to greater mechanical stretch on endothelial cells and vascular smooth muscle cells. Mechanical stretch causes endothelial cells to release signaling proteins, like platelet-derived growth factor, that stimulate smooth muscle cell proliferation (Hu et al. 1998). In response to increased mechanical stretch, vascular smooth muscle cells stimulate collagen production (Durante et al. 2000; Solan et al. 2009). Together, smooth muscle cell proliferation and collagen deposition increase arterial stiffness and protect the artery from the chronic wall strain induced by elevated PP. However, increased cerebral artery stiffness will also lead to transmission of the elevated PP further into the cerebral circulation, potentially damaging the more fragile arterioles and capillaries (Mitchell 2018). Thus, age-related increases in PP may induce arterial remodeling that has both physiologic and pathophysiologic consequences.
The goal of this project was to examine the interaction of age and PP on cerebral artery endothelial function. We hypothesized that high PP would cause a larger impairment in endothelium-dependent dilation in posterior cerebral arteries (PCAs) from old mice compared with young mice. We further hypothesized that exposure to high PP would result in lower NO-mediated vasodilation, measured by the change in endothelium-dependent vasodilation after inhibition of NO synthase (NOS) by N(ω)-nitro-L-arginine methyl ester (L-NAME). We also examined the role of hydrogen peroxide in the endothelium-dependent vasodilation response by incubation with catalase. Additionally, we hypothesized old cerebral arteries would be more stiff and distend less to high PP compared with young cerebral arteries. To test these hypotheses, we collected PCAs from young and old male C57BL/6 mice. The isolated PCAs were exposed ex vivo to static pressure, low PP, and high PP, followed by measurement of endothelial function.
2. Materials and methods
2.1. Animals
Young (n=37, 5–10 months) and old (n=25, 24–30 months) male C57BL/6 mice were housed at the University of Oregon. Mice were obtained from the National Institute on Aging colony at Charles River (old) or purchased from Charles River (young). All mice were housed under a 12/12-hour light-dark cycle at 24°C and provided food ad-libitum. Mice were euthanized via exsanguination under inhaled isoflurane. All protocols were undertaken in compliance with the University of Oregon Institutional Animal Care and Use Committee regulations.
2.2. Ex vivo pulse pressure application
PCA endothelium-dependent dilation was assessed following exposure to pulsation ex vivo. After euthanasia, the PCA was dissected from the brain and placed in a 112PP pulsatile pressure myograph chamber (Danish Myo Technology, Hinnerup, Denmark) containing a physiological salt solution with 145 mM NaCl, 4.7 mM KCl, 2 mM CaCl2, 1.17 mM MgSO4, 1.2 mM NaH2PO4, 5.0 mM glucose, 2.0 mM pyruvate, 0.02 mM EDTA, 3.0 mM MOPS buffer, 10 g/L BSA, 7.4 pH at 37°C. The artery was then fitted over two glass micropipette tips and held in place by nylon suture and perfused with physiological salt solution before being slowly pressurized to 50 mmHg. To measure the effect of pulsation, arteries were pulsed at a frequency of 400 beats per minute at a 50% duty cycle for 30 minutes. The “low PP” condition cycled between 50 and 75 mmHg and the “high PP” condition cycled between 37.5 and 87.5 mmHg, such that mean arterial pressure was similar between conditions (62.5 mm Hg). As the exact PP in the PCA is unknown, we estimated these pressures based on the carotid artery PP in a mouse model of higher large artery stiffness (Walker et al. 2015).
2.3. Endothelial function
Endothelium-dependent dilation was assessed as previously described (Walker et al. 2015). Briefly, the PCA was pre-constricted with phenylephrine (2–8 μM to achieve <80% of baseline lumen diameter) and the change in lumen diameter was measured in response to increasing concentrations of acetylcholine (ACh, 10−9 to 10−4 M). The dose response to ACh was measured during static pressure (50 mmHg) and immediately following the low or high PP conditions. Measurement of NO-dependent dilation was achieved by incubation of the artery in L-NAME (0.1 mM) 30 minutes prior to an ACh dose response. To measure the effect of the removal of hydrogen peroxide, arteries were incubated with PEG-catalase (100 U/ml) for 1 hour prior to a dose response. Dose-dependent responses to incubation with the endothelium-independent dilator sodium nitroprusside (SNP, 10−9 to 10−3 M) were measured in old and young arteries after exposure to high PP.
2.4. Arterial distension with pulsatile pressure application
To assess the change in PCA lumen diameter during the application of pulsatile pressure, 30-second, high frame rate video recordings were acquired with MyoView software (Danish Myo Technology, Hinnerup, Denmark) following 30 minutes of pulsation. Frame-by-frame diameters were measured and averaged across five distinct arterial sections with VasoTracker Image Analyzer (open source). To exclude anomalies in the diameter tracking, the reported maximum and minimum diameters had to occur in greater than 10% of the total frames. Absolute distension was calculated as Dmax-Dmin, and percent distension as (Dmax-Dmin)/Dmax *100, where Dmax is the maximum lumen diameter and Dmin is the minimum lumen diameter.
2.5. Arterial stiffness calculation
Using the artery distension during the application of pulsatile pressure, PCA stiffness was calculated. β stiffness index was calculated as ln(Phigh/Plow)/(Dmax− Dmin)/Dmin, where Phigh is the highest pressure and Plow is the lowest pressure during the pulse application (Flamant et al. 2007). Peterson modulus of elasticity (Ep) was calculated as Dmin*(Phigh – Plow)/(Dmax-Dmin)(Leloup et al. 2016).
2.6. Statistical analysis
Statistical analyses were performed with R (version 4.0.5), IBM SPSS (version 24, Armonk, NY) or SAS 9.4 version (SAS Institute Inc., Cary, NC, USA). Data were checked for normality with a Shapiro-Wilk test. For dose responses to ACh and ACh+LNAME, to address the change of the outcome variable (vasodilation), we employed a mixed effects model of which repeated ANOVA is a special case. In this model, we included dose and pulse as our fixed main effects, and their interaction term dose*pulse as well. A subject-specificity was treated as a random effect in the model, to capture the correlation within a subject over the dose response. Overall F-test statistics for each main effect and interaction are reported. Post-hoc comparisons were further examined with Tukey multiple comparison procedure, controlled with a family-wise error rate of 0.05. For the dose response to SNP, a similar mixed effects repeated ANOVA analysis was conducted, but with the two fixed main effects of group (young vs. old) and dose condition. To compare the maximal response to ACh between young and old for pulse conditions, a similar mixed effects repeated ANOVA analysis was conducted, but with the two fixed main effects of group (young vs. old) and pulse condition. The effect of catalase on vasodilation was assessed by independent t-tests comparing maximal ACh vs. ACh+catalase conditions within an age and pulse condition, as these data are not matched between conditions. Comparisons of distension, stiffness, pre-constriction and spontaneous tone between age groups were performed by t-tests for independent samples, except for β-stiffness and Ep which were assessed by Mann-Whitney U test as these data were not normally distributed. . EC50’s were calculated by fitting each dose response to a 4-parameter logistic equation, and the comparison of young vs. old was made by independent samples t-test. Significance was set at P<0.05. Values are presented as mean ± SD.
3. Results
3.1. Cerebral artery endothelial function after pulsatile pressure exposure
In PCAs from young mice, the vasodilation to ACh was significantly different with dose (p<0.0001), pulse condition (p<0.0001), and the interaction of dose × pulse condition was significant (p=0.023, Figure 1A). In young PCAs, there was no significant difference in the response to ACh between the low PP and the static pressure conditions (dose-response RM-ANOVA post-hoc: p=0.43, maximal response RM-ANOVA post-hoc: p=0.99, Figure 1A,E). However, when exposed to a high PP, young PCAs had a lower response to ACh compared with the static pressure condition (dose-response RM-ANOVA post-hoc: p<0.001, maximal response RM-ANOVA post-hoc: p=0.003) and low PP condition (dose-response RM-ANOVA post-hoc: p<0.001, maximal response RM-ANOVA post-hoc: p=0.006, Figure 1A,E). These results indicate that high PP impairs the vasodilatory response to ACh in young cerebral arteries.
Figure 1. High pulse pressure impairs endothelial function in cerebral arteries from young mice, but not old mice.
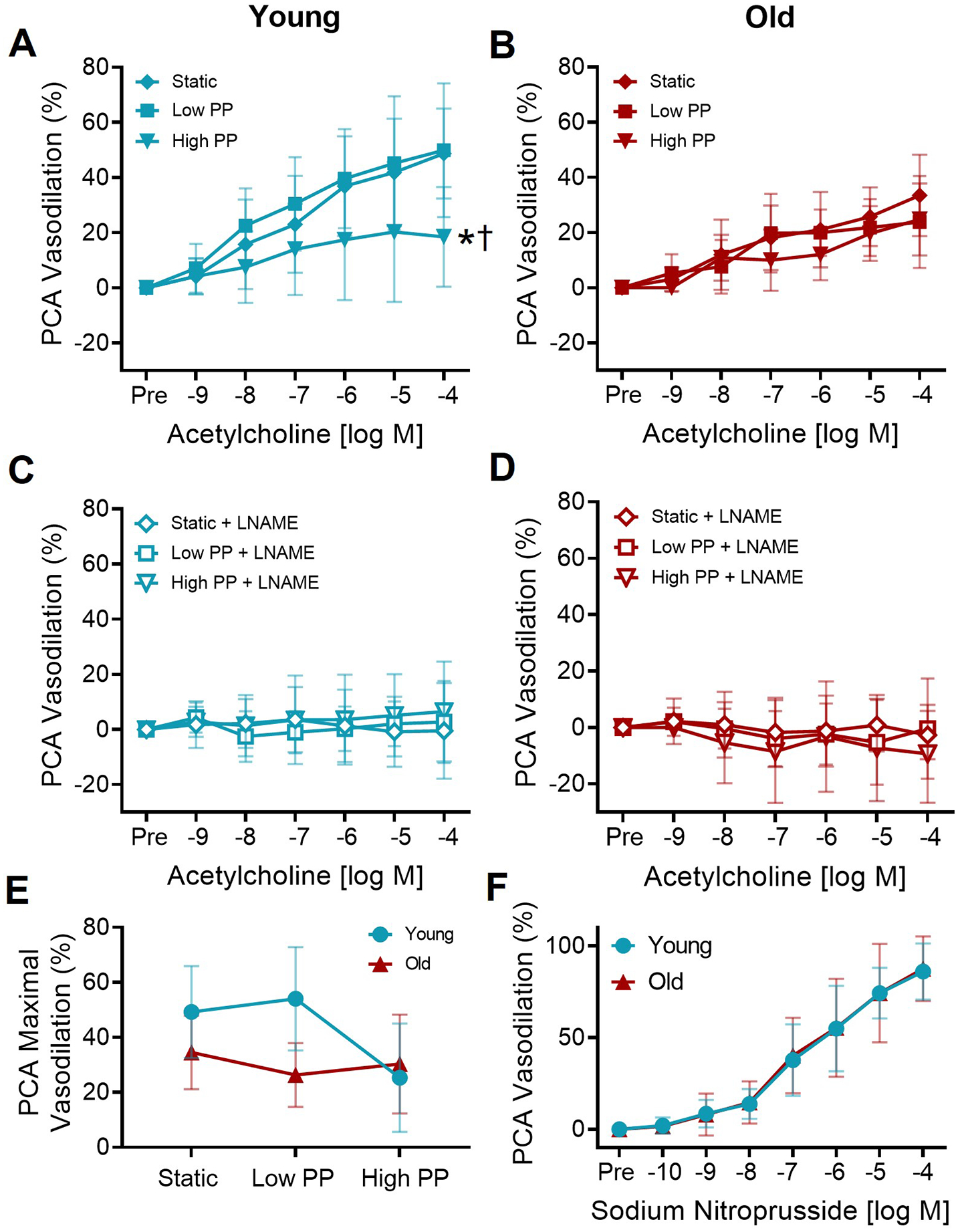
The dose-dependent dilation in (A) young and (B) old posterior cerebral arteries (PCAs) to increasing concentrations of acetylcholine following exposure to static pressure, low pulse pressure (PP), and high PP. In young PCAs, there was a significant ACh dose × PP interaction (p=0.02). There was no ACh dose × PP interaction in the old PCAs. n=6–20/group *p<0.05 high PP vs. static, †p<0.05 high PP vs. low PP, Tukey post-hoc test following significant dose × pulse interaction with RM-ANOVA.. In the presence of nitric oxide synthase inhibitor L-NAME, the dose-dependent dilation in (C) young and (D) old PCAs to increasing concentrations of acetylcholine. n=5–8/group, no interaction of dose × pulse by RM-ANOVA. (C). Maximal dilation to acetylcholine in young and old PCAs following static pressure, low PP, and high PP. n=6–20/group. (D). The dose-dependent dilation of young and old PCAs to increasing concentrations of sodium nitroprusside in PCAs. n=10–11/group, no interaction of dose × age by RM-ANOVA. Values are mean ± SD.
In PCAs from old mice, there was no significant interaction of dose × pulse condition (p=0.19) for dose-dependent vasodilation responses to ACh (Figure 1B). Thus, the pulse pressure conditions did not affect the response to ACh in old cerebral arteries. In the old PCAs, there was a significant effect of pulse condition for the ACh response (p=0.02) that appears to be driven by the lower doses, as there is no difference in maximal ACh response between pulse condition (p=0.14). In old PCAs, there was no significant difference in the response to ACh between static pressure and low PP (dose-response RM-ANOVA post-hoc: p=0.19, maximal response RM-ANOVA post-hoc: p=0.99) or high PP (dose-response RM-ANOVA post-hoc: p=0.44, maximal response RM-ANOVA post-hoc: p=0.92, Figure 1B,E). There was a significant difference in the between low PP and high PP for the dose response, but not the maximal response (dose-response RM-ANOVA post-hoc: p=0.02, maximal response RM-ANOVA post-hoc: p=0.99, Figure 1B,E). When comparing the maximal vasodilation to ACh between age groups, there was a significant effect of both age (p=0.004) and pulse condition (p=0.007), but no interaction of age × pulse condition (p=0.14, Figure 1E).
Endothelium-independent dilation, measured by the dose-dependent vasodilation response to SNP, was not different between young and old PCAs (interaction dose × group: p=0.99, Figure 1F). For ACh and SNP dose responses, there was no difference in sensitivity (EC50), spontaneous tone, or preconstriction between young and old for each treatment condition, nor differences in maximal artery diameters (all p>0.05, Supplemental Tables 1–3). To summarize, exposure to a high PP resulted in impaired endothelium-dependent dilation in young cerebral arteries, but not old cerebral arteries.
3.2. NO-mediated vasodilation
In both young and old PCAs after incubation with L-NAME, the vasodilation to ACh was not significantly affected by dose (young: p=0.89, old: p=0.71) or the interaction of dose × pulse condition (young: p=0.95, old: p=0.93, Figure 1C,D). There was a significant effect of the pulse condition in the PCAs after L-NAME incubation (young: p=0.01, old: p=0.02), but no significant difference in maximal ACh response (young: p=0.63, old: p=0.60). Furthermore, within each age and pulse condition, the maximal response to ACh was lower in the presence compared with the absence of L-NAME (p<0.05), with vasodilation in response to ACh being nearly absent in the presence of L-NAME in most cases. These results indicate that NO was the major mediator of endothelium-dependent vasodilation, and the bioavailability of NO is reduced with exposure to high PP in young cerebral arteries.
3.3. Effect of catalase on endothelial function after pulsatile pressure exposure
In young PCAs under static pressure, incubation with PEG-catalase did not alter the maximal vasodilation to ACh (p=0.19). In contrast, following low PP in young PCAs, the maximal vasodilation to ACh was lower in the presence of PEG-catalase compared to the absence of PEG-catalase (p<0.001). After exposure to high PP in young PCAs, there was a greater maximal response to ACh with PEG-catalase compared to without PEG-catalase (p=0.01, Figure 2A). These results demonstrate that catalase impairs endothelium-dependent vasodilation during low PP and augments endothelium-dependent vasodilation during high PP in young cerebral arteries.
Figure 2. Age and pulse pressure modulate the contribution of reactive oxygen species to endothelium-dependent dilation.
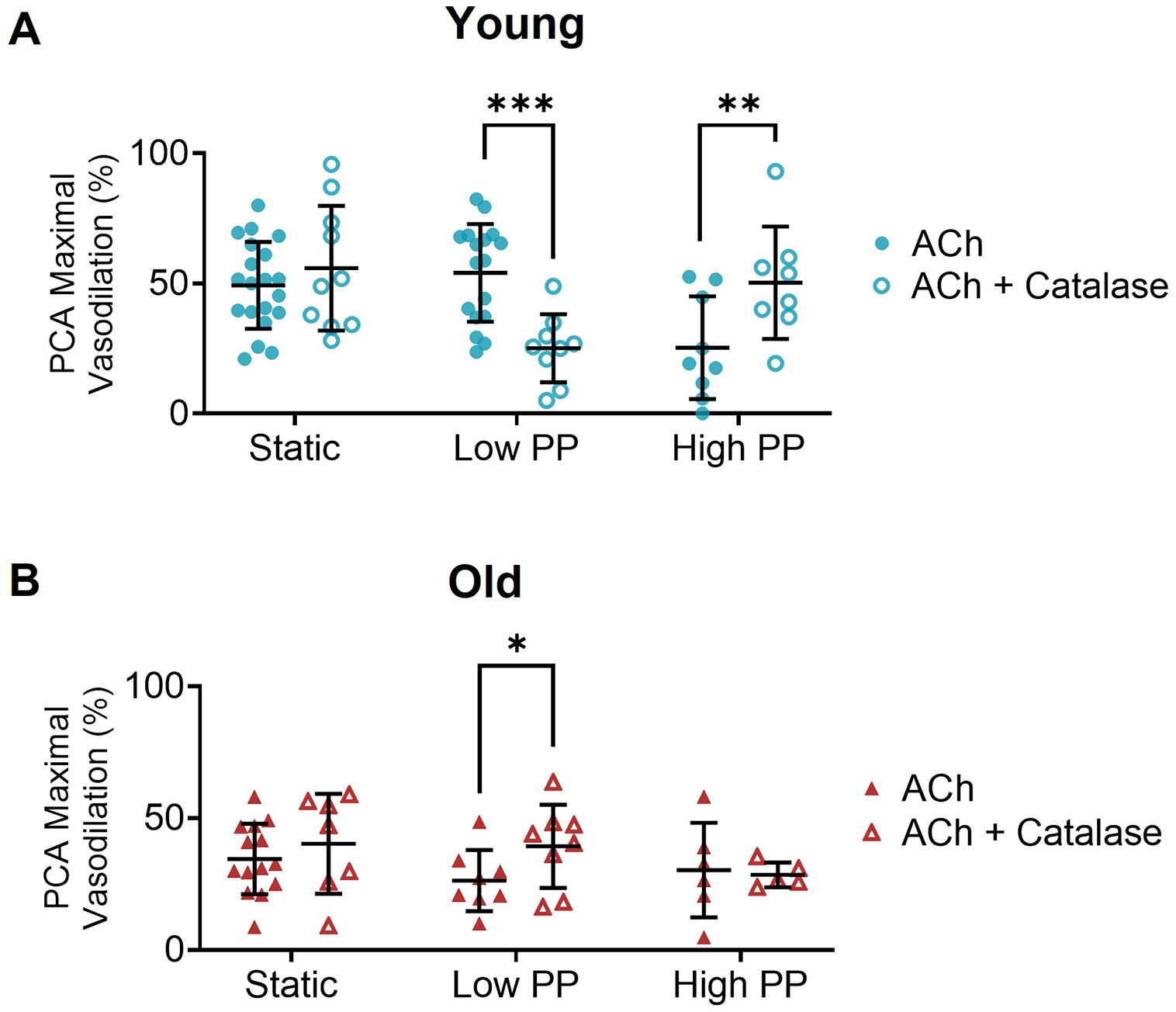
In (A) young and (B) old posterior cerebral arteries (PCAs) the maximal response to acetylcholine in the absence and presence of PEG-catalase following static pressure, low pulse pressure (PP), and high PP exposure. n=6–20/group, *p<0.05, **p<0.01, ***p<0.001 with vs. without PEG-catalase assessed by independent samples t-tests within same pressure condition. Values are mean ± SD.
In old PCAs under static and high PP conditions, incubation with PEG-catalase did not alter the maximal ACh response (static: p=0.21, high PP: p=0.28). In contrast, in old PCAs after exposure to low PP, PEG-catalase resulted in a greater maximal ACh response compared to the response without PEG-catalase (p=0.04, Figure 2B). Thus, in contrast to the young cerebral arteries, catalase augments endothelium-dependent vasodilation during low PP in old cerebral arteries.
3.4. Cerebral artery distension and stiffness during pulsatile pressure exposure
At low PP, there was no significant difference in distension between young and old PCAs, when expressed as either an absolute change in diameter (p=0.42) or percent change in diameter (p=0.30). In contrast, PCAs from young mice displayed greater distension compared with old mice at high PP (absolute change in diameter, p=0.02; percent change in diameter, p=0.02; Figure 3A, Supplemental Table 4). At low PP, there was no significant difference in PCA stiffness between young and old, when measured either as β-stiffness index (p=0.18) or Ep (p=0.18). In contrast, at high PP, old PCAs were stiffer than young PCAs for both β-stiffness index (p=0.032) and Ep (p=0.032, Figure 3B, Supplemental Table 4). Differences in arterial tone do not appear to contribute to the age-related difference in distension as spontaneous tone was not significantly different between age groups following exposure to low and high PP, or at static pressure (p>0.05, Supplemental Table 1).
Figure 3. Lower distension and greater stiffness in cerebral arteries with advanced age.
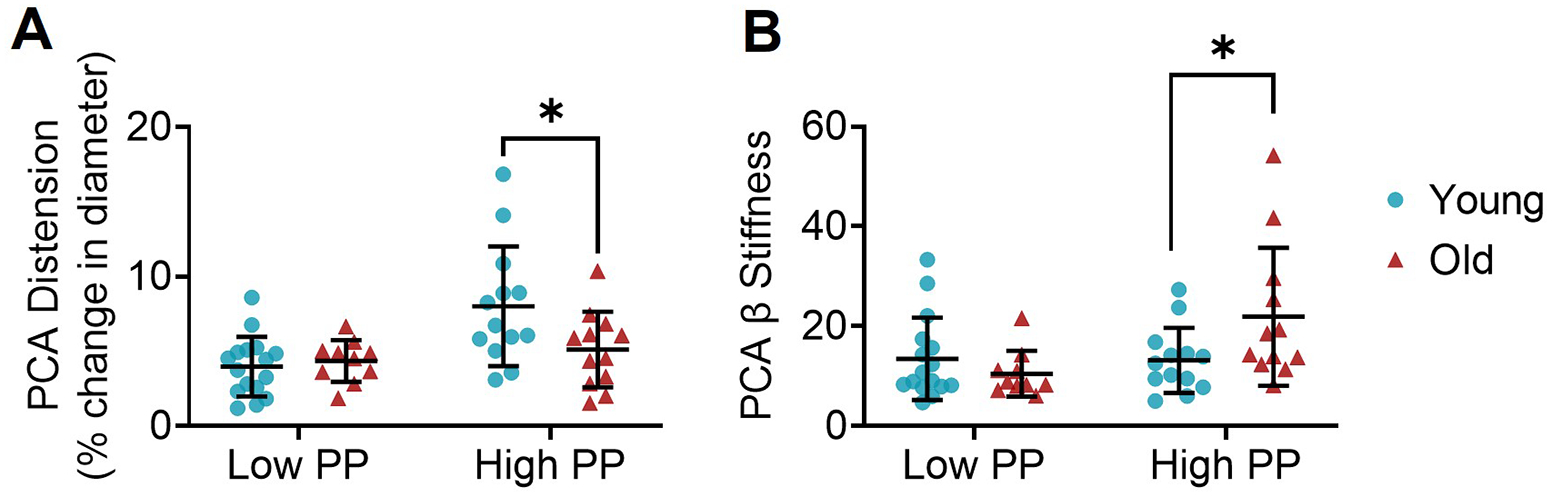
(A) Distension of the posterior cerebral artery (PCA) from young and old mice expressed as the percent change in diameter during low pulse pressure (PP) and high PP application. (B) β-stiffness index in PCAs from young and old mice during low PP and high PP application. n=10–16/group. *p<0.05 young vs. old within pulse condition, assessed by independent samples t-tests. Values are mean ± SD.
4. Discussion
The results of this study demonstrate, for the first time, that there are age-related differences in the cerebral artery endothelial cell response to acute high PP. We hypothesized that old cerebral arteries would be more vulnerable to the negative effects of high PP, but we found the opposite. We find that ex vivo exposure to high PP impairs endothelium-dependent dilation in young cerebral arteries, but does not affect old cerebral arteries. Our results further indicate that reduced NO bioavailability and increased ROS are mechanisms for impaired endothelial function at high PP in young cerebral arteries. We also found that old cerebral arteries have less distension during exposure to high pulse pressure and greater stiffness. The higher stiffness of the old cerebral arteries is a possible mechanism for the protection against high PP-induced endothelial dysfunction. These results are important as they suggest that cerebral arteries adapt to increases in PP with advancing age, but the potentially harmful consequences of this adaptation need further exploration.
4.1. Impaired cerebral artery endothelial function with advancing age
Our observations at static pressure indicate impaired cerebral artery endothelial function with advancing age, similar to previous studies (Modrick et al. 2009b; Toth et al. 2014; Walker et al. 2014). The age-related decline in vasodilation to ACh arises from endothelial cell dysfunction as there was no difference in endothelium-independent dilation between age groups. We found that PCA vasodilation to ACh is almost completely mediated by NOS as nearly all vasodilation was inhibited by L-NAME for both young and old, similar to previous findings (Modrick et al. 2009a; Walker et al. 2014). These results indicate that NO is the major mediator of cerebral artery endothelium-dependent dilation, and decreased bioavailability of NO is the primary mediator of impaired cerebral artery endothelial function with advancing age. Previous studies suggest that superoxide and Rho kinase contribute to the reduced cerebral artery NO bioavailability with advancing age (De Silva et al. 2018; Walker et al. 2014). Similar to aging, mouse models of greater large artery stiffness also have impaired resistance artery endothelial function due to excess superoxide (Walker et al. 2015) and increased resistance artery vasoconstriction due to the Rho kinase pathway (Osei-Owusu et al. 2014). Thus, advanced age and greater large artery stiffness are associated with a decline in cerebral artery endothelial function that is attributable to decreased NO bioavailability and excess superoxide.
4.2. High pulse pressure and cerebral artery endothelial dysfunction
The cerebrovascular impairments associated with greater large artery stiffness are assumed to be mediated by increases in PP (Thorin-Trescases et al. 2018). Pulsatile blood pressure or flow at low amounts have generally favorable effects on endothelial cells (Raignault et al. 2017; Thacher et al. 2010). In agreement with a previous study, we find that young cerebral arteries have impaired endothelial function after exposure to high PP (Raignault et al. 2017). Yet, there was no decline in endothelium-dependent dilation in the old cerebral arteries with high PP. We do not believe the results in the old cerebral arteries are attributable to a “floor effect” as at high PP these arteries have reduced vasodilation to ACh in the presence of L-NAME, suggesting that there remains some NO-mediated dilation after exposure to high PP. Also of note, we did not find an improvement in endothelial function with low PP compared with static pressure, unlike previous studies (Raignault et al. 2017; Thacher et al. 2010), perhaps due to the short duration of our pulse exposure. The value we chose for the low PP condition is similar to the PP measured in vivo in young C57BL6 mice (Kawarazaki et al. 2020; Wirth et al. 2016). While we based our values for the high PP condition on the in vivo PP in a mouse model of elevated large artery stiffness (Walker et al. 2015), we acknowledge that our high PP condition is higher than that of an old C57BL6 mouse (DuPont et al. 2016; Kawarazaki et al. 2020; Wirth et al. 2016). Furthermore, this was a very acute exposure to high PP, and future studies should examine the impact of longer-term exposures. Our results imply that young cerebral arteries are susceptible to the negative effects of a high PP, while old cerebral arteries are protected against these effects.
4.3. Mechanisms for impaired endothelial function with high pulse pressure
Our results indicate distinct mechanisms for endothelium-dependent dilation under different pressure conditions. Increases in PP augment the circumferential stretch on the arterial cells (Anwar et al. 2012) and ROS production is increased when stretch is applied to cultured endothelial cells (Cheng et al. 2011). Previous research by Raignault, et al. described differing mechanisms for endothelium-dependent dilation under static and pulsatile pressure conditions in cerebral arteries from young mice. Their findings suggest that hydrogen peroxide contributes to both cerebral artery flow-mediated dilation and ACh-mediated dilation at static pressure as these responses are attenuated by catalase (Raignault et al. 2017). They further demonstrate that exposure to pulsatile pressure impairs flow-mediated dilation and ACh-mediated dilation, a response mediated by ROS as indicated by an improvement in the responses with catalase present (Raignault et al. 2017). Our results differ from this previous study in that catalase did not reduce vasodilation in the static pressure condition. Conversely, we find that catalase impairs endothelium-dependent dilation after exposure to low PP in young cerebral arteries. We also find that after exposure to high PP, endothelium-dependent dilation of young cerebral arteries is impaired by ROS as suggested by an improvement with catalase. The discrepancy in these findings could be due to differing PP magnitude and/or delivery, or differences in oxygen tension that are known to alter the contribution of hydrogen peroxide to vasodilation (Drouin et al. 2007). Our results at static pressure also agree with previous findings that found hydrogen peroxide does not mediate cerebral artery vasodilation to acetylcholine (Modrick et al. 2009a). A novel aspect of our study is the inclusion of old mice. We find slight improvements with catalase in the old cerebral arteries after exposure to low PP, and this may indicate increased ROS production in this condition, but this area needs further investigation. Thus, our results suggest that hydrogen peroxide contributes to endothelium-dependent dilation after low PP in young but not old cerebral arteries, and that ROS impairs endothelium-dependent dilation at high PP in cerebral arteries from young mice.
4.4. Age-related changes to cerebral artery stiffness
The lack of an effect of high PP on cerebral arteries from old mice suggests that there are potential age-related changes to arteries that are protective. Increased stiffness will protect the wall against added stretch at high PP. Indeed, we find that at high PP, old cerebral arteries distend less and are more stiff than the young cerebral arteries. With the measures in our study, we cannot determine the contributions of active vs. passive factors to the age-related increase in cerebral artery stiffness. Previous studies have reported greater passive stiffness in old cerebral arteries compared with young (Diaz-Otero et al. 2016). Active contributors to stiffness could be reduced vasodilator tone (e.g., less NO bioavailability) or increased vasoconstrictor tone. We find no difference in spontaneous tone after exposure to pulsatile pressure between young and old cerebral arteries in this study, and this may indicate no difference in the active contributors to stiffness but is not definitive proof. Relatedly, old cerebral arteries have a decline in the myogenic response when exposed to cyclic pulsatile pressure (Springo et al. 2015). Taken together, these findings suggest that passive factors contribute to the age-related increase in cerebral artery stiffness.
This increased cerebral artery stiffness with age could have devasting consequences on the brain. First, the greater stiffness of the major cerebral arteries delivers the elevated PP further into the cerebrovascular tree, potentially damaging the microvasculature (Mitchell 2018). Second, extension of this greater stiffness into the cerebral arterioles, resulting in reduced distension, could lead to less clearance of interstitial fluid along the paravascular pathway (Iliff et al. 2013). For example, old mice have less distension of cortical arterioles and an impaired clearance of parenchymal amyloid-β (Kress et al. 2014). If these adaptations of the cerebral vasculature to high PP with aging are irreversible, then this may explain why mid-life hypertension is more predictive of cognitive impairment than late-life hypertension (Iadecola et al. 2016). Thus, age-related increases in cerebral artery stiffness may protect those arteries from further damage by elevated PP, but potentially impairs the cerebral microvasculature and decreases the clearance of amyloid-β, increasing the risk factors for dementia.
4.5. Pulsatile flow and endothelial cell function
In addition to changing circumferential stress on the artery wall, increases in PP will also result in increases in blood flow pulsatility (Mitchell 2018). In general, pulsatile flow is beneficial for endothelial cells compared with non-pulsatile (steady) flow, with pulsatile flow leading to more NO production (Nakano et al. 2000; Noris et al. 1995). However, high amounts of flow pulsatility appear detrimental and increase endothelial cell inflammatory signaling and ROS production (Li et al. 2013; Silacci et al. 2001). Old age is associated with a reduced mechanosensing of wall shear stress (Tian et al. 2022), and this potentially leads to the lack of a negative impact of high PP in old arteries in this study. Counter to this hypothesis, we previously demonstrated that age does not affect the arterial response to blood flow oscillations, demonstrated by young and old mice having similar pro-atherogenic responses to increased oscillatory blood flow (Walker et al. 2019). A limitation of our method for the present study is that fluid movement into and out of the artery is needed in order to create pulsatile intraluminal pressure. Thus, in this study we cannot disentangle the contributions of pulsatile pressure and flow. Given the relationship between pressure and flow, most studies (including ours) are not able to determine the respective contributions of pulsatile pressure compared with pulsatile flow on endothelial dysfunction or disease.
4.6. Limitations
There are a few limitations of this study. First, only male mice were studied. There are sex differences in the relations of PP with brain outcomes, as well as with the relation of cerebral artery dysfunction to biological aging (Cole et al. 2021; Kehmeier and Walker 2021). Thus, it will be important to examine the effects of elevated PP on cerebral arteries from females in future studies. We also studied only two age groups, and therefore, do not know the trajectory of these changes in middle-age (The Journal of Neuroscience 2019). While the low PP condition we chose is similar to the PP in a young wildtype mouse, our high PP condition is above that found with normal aging in mice (DuPont et al. 2016; Kawarazaki et al. 2020; Wirth et al. 2016). In addition, the mean pressure for the pulse and static conditions may be lower than in vivo mouse cerebral artery pressures (Mayhan et al. 1986). We also used phenylephrine to pre-constrict the arteries before performing dose responses to vasodilators. The use of phenylephrine, rather than relying on spontaneous tone, could have introduced changes to the cellular signaling. Lastly, we chose a pulse rate of 400 beats per minute. While this chosen pulse rate matches the heart rate of thermoneutral unrestrained C57BL6 mice, the mice in this study were housed at 21°C and thus the in vivo exposure to heart rate was likely about 200 beats per minute higher than our ex vivo pulse rate (Axsom et al. 2020).
5. Conclusions
The results of this study provide important new insights into the effects of PP on cerebrovascular function, and the mediating effects of age on this interaction. We find that an acute exposure to a high PP impairs endothelial function in cerebral arteries from young mice. In contrast, old cerebral arteries are protected from the damaging effects of acute exposure to high PP. An age-related increase in stiffness likely protects the old cerebral arteries from the negative consequences of exposure to high PP. However, this protection potentially comes at the expense of downstream damage due to a greater transmission of elevated PP to the cerebral microvasculature. Ultimately, these results support that more attention is needed to the benefits of preventing the age-related increases in PP.
Supplementary Material
Highlights.
High pulse pressure impairs cerebral artery endothelial function in young mice.
Old cerebral arteries are protected against damage by high pulse pressure.
Old cerebral arteries are stiffer and distend less at high pulse pressure.
Acknowledgments
We would like to thank Jessica LaFarga for her assistance with manuscript citations and editing.
Sources of Funding
This work was supported by National Institutes of Health R01 AG064016 and an Oregon Medical Research Foundation New Investigator Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no conflicts of interest.
References
- Anwar MA; Shalhoub J; Lim CS; Gohel MS; Davies AH The effect of pressure-induced mechanical stretch on vascular wall differential gene expression. J Vasc Res. 49:463–478; 2012 [DOI] [PubMed] [Google Scholar]
- Axsom JE; Nanavati AP; Rutishauser CA; Bonin JE; Moen JM; Lakatta EG Acclimation to a thermoneutral environment abolishes age-associated alterations in heart rate and heart rate variability in conscious, unrestrained mice. Geroscience. 42:217–232; 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel R; Dvir D; Arbel Y; Shechter A; Feinberg MS; Shechter M Pulse pressure is a predictor of vascular endothelial function in middle-aged subjects with no apparent heart disease. Vasc Med. 15:299–305; 2010 [DOI] [PubMed] [Google Scholar]
- Ceravolo R; Maio R; Pujia A; Sciacqua A; Ventura G; Costa MC; Sesti G; Perticone F Pulse pressure and endothelial dysfunction in never-treated hypertensive patients. J Am Coll Cardiol. 41:1753–1758; 2003 [DOI] [PubMed] [Google Scholar]
- Cheng Z; Jiang X; Kruger WD; Praticò D; Gupta S; Mallilankaraman K; Madesh M; Schafer AI; Durante W; Yang X; Wang H Hyperhomocysteinemia impairs endothelium-derived hyperpolarizing factor-mediated vasorelaxation in transgenic cystathionine beta synthase-deficient mice. Blood. 118:1998–2006; 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JA; Kehmeier MN; Bedell BR; Krishna Kumaran S; Henson GD; Walker AE Sex Differences in the Relation Between Frailty and Endothelial Dysfunction in Old Mice. J Gerontol A Biol Sci Med Sci; 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva TM; Modrick ML; Dabertrand F; Faraci FM Changes in Cerebral Arteries and Parenchymal Arterioles With Aging: Role of Rho Kinase 2 and Impact of Genetic Background. Hypertension. 71:921–927; 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Otero JM; Garver H; Fink GD; Jackson WF; Dorrance AM Aging is associated with changes to the biomechanical properties of the posterior cerebral artery and parenchymal arterioles. Am J Physiol Heart Circ Physiol. 310:H365–375; 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ; Morgan RG; Walker AE; Lesniewski LA Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol. 89:122–135; 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin A; Thorin-Trescases N; Hamel E; Falck JR; Thorin E Endothelial nitric oxide synthase activation leads to dilatory H2O2 production in mouse cerebral arteries. Cardiovasc Res. 73:73–81; 2007 [DOI] [PubMed] [Google Scholar]
- DuPont JJ; McCurley A; Davel AP; McCarthy J; Bender SB; Hong K; Yang Y; Yoo JK; Aronovitz M; Baur WE; Christou DD; Hill MA; Jaffe IZ Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging. JCI Insight. 1:e88942; 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante W; Liao L; Reyna SV; Peyton KJ; Schafer AI Physiological cyclic stretch directs L-arginine transport and metabolism to collagen synthesis in vascular smooth muscle. Faseb j. 14:1775–1783; 2000 [DOI] [PubMed] [Google Scholar]
- Ferrucci L; Gonzalez-Freire M; Fabbri E; Simonsick E; Tanaka T; Moore Z; Salimi S; Sierra F; de Cabo R Measuring biological aging in humans: A quest. Aging Cell. 19:e13080; 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamant M; Placier S; Dubroca C; Esposito B; Lopes I; Chatziantoniou C; Tedgui A; Dussaule JC; Lehoux S Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension. 50:212–218; 2007 [DOI] [PubMed] [Google Scholar]
- Hu YH; Bock G; Wick G; Xu QB Activation of PDGF receptor alpha in vascular smooth muscle cells by mechanical stress. Faseb Journal. 12:1135–1142; 1998 [DOI] [PubMed] [Google Scholar]
- Iadecola C; Yaffe K; Biller J; Bratzke LC; Faraci FM; Gorelick PB; Gulati M; Kamel H; Knopman DS; Launer LJ; Saczynski JS; Seshadri S; Zeki Al Hazzouri A; American Heart Association Council on, H.; Council on Clinical, C.; Council on Cardiovascular Disease in the, Y.; Council on, C.; Stroke, N.; Council on Quality of, C.; Outcomes, R.; Stroke, C. Impact of Hypertension on Cognitive Function: A Scientific Statement From the American Heart Association. Hypertension. 68:e67–e94; 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ; Lee H; Yu M; Feng T; Logan J; Nedergaard M; Benveniste H Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 123:1299–1309; 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katusic ZS; Marshall JJ; Kontos HA; Vanhoutte PM Similar responsiveness of smooth muscle of the canine basilar artery to EDRF and nitric oxide. Am J Physiol. 257:H1235–1239; 1989 [DOI] [PubMed] [Google Scholar]
- Kawarazaki W; Mizuno R; Nishimoto M; Ayuzawa N; Hirohama D; Ueda K; Kawakami-Mori F; Oba S; Marumo T; Fujita T Salt causes aging-associated hypertension via vascular Wnt5a under Klotho deficiency. J Clin Invest. 130:4152–4166; 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehmeier MN; Walker AE Sex Differences in Large Artery Stiffness: Implications for Cerebrovascular Dysfunction and Alzheimer’s Disease. Front Aging. 2; 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress BT; Iliff JJ; Xia M; Wang M; Wei HS; Zeppenfeld D; Xie L; Kang H; Xu Q; Liew JA; Plog BA; Ding F; Deane R; Nedergaard M Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 76:845–861; 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leloup AJ; Van Hove CE; Kurdi A; De Moudt S; Martinet W; De Meyer GR; Schrijvers DM; De Keulenaer GW; Fransen P A novel set-up for the ex vivo analysis of mechanical properties of mouse aortic segments stretched at physiological pressure and frequency. J Physiol. 594:6105–6115; 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M; Tan Y; Stenmark KR; Tan W High Pulsatility Flow Induces Acute Endothelial Inflammation through Overpolarizing Cells to Activate NF-kappaB. Cardiovasc Eng Technol. 4:26–38; 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhan WG; Faraci FM; Baumbach GL; Heistad DD Effects of aging on responses of cerebral arterioles. Am J Physiol. 258:H1138–1143; 1990 [DOI] [PubMed] [Google Scholar]
- Mayhan WG; Faraci FM; Heistad DD Disruption of the blood-brain barrier in cerebrum and brain stem during acute hypertension. Am J Physiol. 251:H1171–1175; 1986 [DOI] [PubMed] [Google Scholar]
- McEniery CM; Wallace S; Mackenzie IS; McDonnell B; Yasmin; Newby DE; Cockcroft JR; Wilkinson IB Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension. 48:602–608; 2006 [DOI] [PubMed] [Google Scholar]
- Mitchell GF Aortic stiffness, pressure and flow pulsatility, and target organ damage. J Appl Physiol (1985). 125:1871–1880; 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrick ML; Didion SP; Lynch CM; Dayal S; Lentz SR; Faraci FM Role of hydrogen peroxide and the impact of glutathione peroxidase-1 in regulation of cerebral vascular tone. J Cereb Blood Flow Metab. 29:1130–1137; 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrick ML; Didion SP; Sigmund CD; Faraci FM Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol Heart Circ Physiol. 296:H1914–1919; 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T; Tominaga R; Nagano I; Okabe H; Yasui H Pulsatile flow enhances endothelium-derived nitric oxide release in the peripheral vasculature. Am J Physiol Heart Circ Physiol. 278:H1098–1104; 2000 [DOI] [PubMed] [Google Scholar]
- Noris M; Morigi M; Donadelli R; Aiello S; Foppolo M; Todeschini M; Orisio S; Remuzzi G; Remuzzi A Nitric oxide synthesis by cultured endothelial cells is modulated by flow conditions. Circ Res. 76:536–543; 1995 [DOI] [PubMed] [Google Scholar]
- Osei-Owusu P; Knutsen RH; Kozel BA; Dietrich HH; Blumer KJ; Mecham RP Altered reactivity of resistance vasculature contributes to hypertension in elastin insufficiency. Am J Physiol Heart Circ Physiol. 306:H654–666; 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C; Winblad B; Viitanen M; Fratiglioni L Pulse pressure and risk of Alzheimer disease in persons aged 75 years and older: a community-based, longitudinal study. Stroke. 34:594–599; 2003 [DOI] [PubMed] [Google Scholar]
- Raignault A; Bolduc V; Lesage F; Thorin E Pulse pressure-dependent cerebrovascular eNOS regulation in mice. J Cereb Blood Flow Metab. 37:413–424; 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silacci P; Desgeorges A; Mazzolai L; Chambaz C; Hayoz D Flow pulsatility is a critical determinant of oxidative stress in endothelial cells. Hypertension. 38:1162–1166; 2001 [DOI] [PubMed] [Google Scholar]
- Solan A; Dahl SL; Niklason LE Effects of mechanical stretch on collagen and cross-linking in engineered blood vessels. Cell Transplant. 18:915–921; 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springo Z; Toth P; Tarantini S; Ashpole NM; Tucsek Z; Sonntag WE; Csiszar A; Koller A; Ungvari ZI Aging impairs myogenic adaptation to pulsatile pressure in mouse cerebral arteries. J Cereb Blood Flow Metab. 35:527–530; 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S; Virdis A; Ghiadoni L; Salvetti G; Bernini G; Magagna A; Salvetti A Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 38:274–279; 2001 [DOI] [PubMed] [Google Scholar]
- Tarumi T; Thomas BP; Wang C; Zhang L; Liu J; Turner M; Riley J; Tangella N; Womack KB; Kerwin DR; Cullum CM; Lu H; Vongpatanasin W; Zhu DC; Zhang R Ambulatory pulse pressure, brain neuronal fiber integrity, and cerebral blood flow in older adults. J Cereb Blood Flow Metab. 39:926–936; 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacher T; Gambillara V; da Silva RF; Silacci P; Stergiopulos N Reduced cyclic stretch, endothelial dysfunction, and oxidative stress: an ex vivo model. Cardiovasc Pathol. 19:e91–98; 2010 [DOI] [PubMed] [Google Scholar]
- The Journal of Neuroscience, Editorial Board. Considerations for Design of Studies of Normal Aging, Accelerated Aging, and Neurodegeneration. J Neurosci. 39:7032–7033; 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorin-Trescases N; de Montgolfier O; Pincon A; Raignault A; Caland L; Labbe P; Thorin E Impact of pulse pressure on cerebrovascular events leading to age-related cognitive decline. Am J Physiol Heart Circ Physiol. 314:H1214–H1224; 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y; Fopiano KA; Buncha V; Lang L; Rudic RD; Filosa JA; Dou H; Bagi Z Aging-induced impaired endothelial wall shear stress mechanosensing causes arterial remodeling via JAM-A/F11R shedding by ADAM17. Geroscience. 44:349–369; 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P; Tarantini S; Tucsek Z; Ashpole NM; Sosnowska D; Gautam T; Ballabh P; Koller A; Sonntag WE; Csiszar A; Ungvari Z Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol. 306:H299–308; 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao CW; Seshadri S; Beiser AS; Westwood AJ; Decarli C; Au R; Himali JJ; Hamburg NM; Vita JA; Levy D; Larson MG; Benjamin EJ; Wolf PA; Vasan RS; Mitchell GF Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 81:984–991; 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE; Breevoort SR; Durrant JR; Liu Y; Machin DR; Dobson PS; Nielson EI; Meza AJ; Islam MT; Donato AJ; Lesniewski LA The pro-atherogenic response to disturbed blood flow is increased by a western diet, but not by old age. Sci Rep. 9:2925; 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE; Henson GD; Reihl KD; Morgan RG; Dobson PS; Nielson EI; Ling J; Mecham RP; Li DY; Lesniewski LA; Donato AJ Greater impairments in cerebral artery compared with skeletal muscle feed artery endothelial function in a mouse model of increased large artery stiffness. J Physiol. 593:1931–1943; 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE; Henson GD; Reihl KD; Nielson EI; Morgan RG; Lesniewski LA; Donato AJ Beneficial effects of lifelong caloric restriction on endothelial function are greater in conduit arteries compared to cerebral resistance arteries. Age (Dordr). 36:559–569; 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand AJ; Macomber AJ; Walker KS; Edwards L; Thomas KR; Bangen KJ; Nation DA; Bondi MW; Alzheimer’s Disease Neuroimaging I Interactive Effects of Pulse Pressure and Tau Imaging on Longitudinal Cognition. J Alzheimers Dis. 89:633–640; 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth A; Wang S; Takefuji M; Tang C; Althoff TF; Schweda F; Wettschureck N; Offermanns S Age-dependent blood pressure elevation is due to increased vascular smooth muscle tone mediated by G-protein signalling. Cardiovasc Res. 109:131–140; 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


