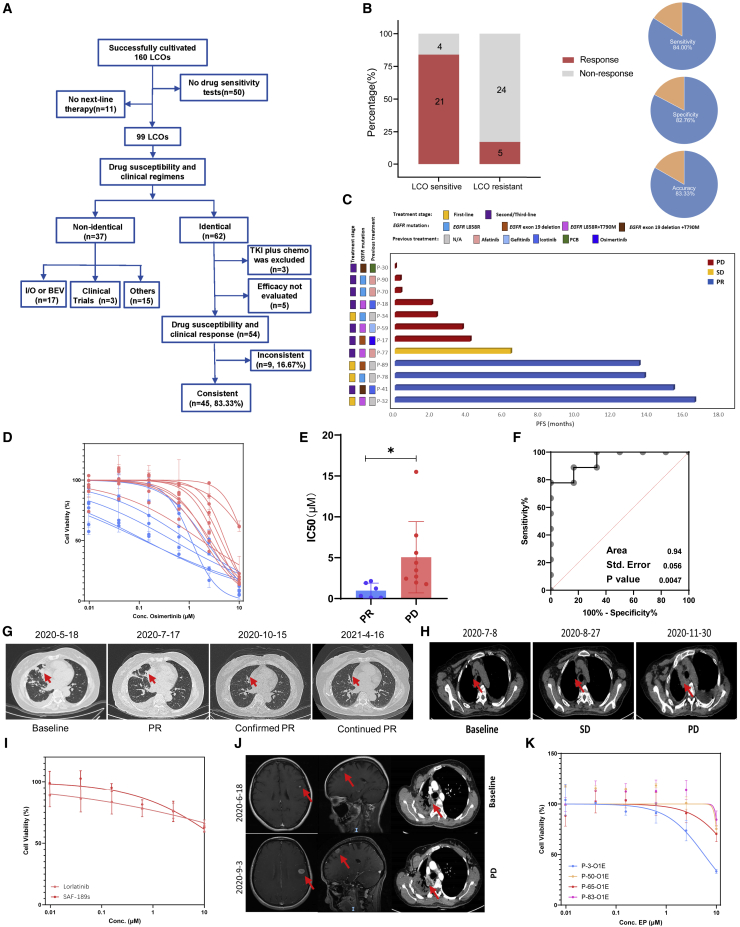
Figure 3.
Comparison of LCO-based drug screening and clinical response
See also Figure S3 and Table S6.
(A) Flowchart of LCO-DST and clinical follow-ups in this study.
(B) The overall correlation between LCO-DST sensitivity and clinical response.
(C) Swimming graph of the progression-free survival (PFS) of patients who received osimertinib treatment.
(D) Dose-effect curves of LCO based on in vitro sensitivity of osimertinib.
(E) Violin plot of the IC50 values of osimertinib for clinical PR and PD groups.
(F) Receiver operating characteristic (ROC) analysis of osimertinib LCO drug tests showed an area under the curve (AUC) of 0.94 with a p value of 0.0047. p values are determined from the normal distribution (two-tail) for the comparison to a chance-level ROC curve (AUC = 0.5).
(G) Computed tomography (CT) scan of P-41 at the baseline, PR, and confirmed PR stages.
(H) CT scan of P-59 at the baseline, SD, and PD stages.
(I) Dose-effect curve of loratinib and SAF-189s for LCOs derived from P-63.
(J) CT scan of the brain and thoracic cavity of P-63 at baseline and PD stages.
(K) Dose-effect curves of the EP regimen (etoposide and paclitaxel) for LCOs derived from P-3, P-50, P-65, P-83.
∗: p <0.05.