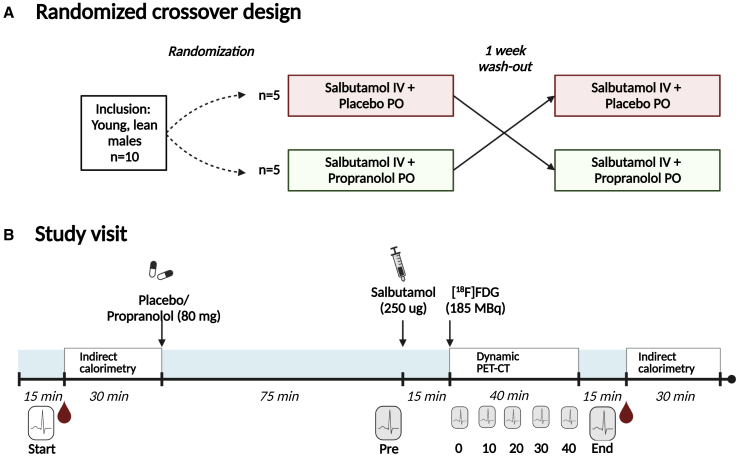
Figure 1.
Study design and timeline of study procedures
(A) This study had a randomized, double-blinded, crossover design.
(B) Both study visits started with the measurement of blood pressure and heart rate (indicated by the ECG icon). Thereafter, the first blood sample (indicated by blood drop icon) was drawn, followed by an indirect calorimetry measurement for 30 min. Then, participants received either placebo or propranolol (80 mg, in two capsules; per oral, PO), depending on the study visit. After 75 min, blood pressure and heart rate were measured again, and a single bolus of salbutamol (250 μg; intravenous, IV) was injected over a continuous time course of 5 min. 15 min after initiation of the injection, a low-dose computed tomography (CT) scan was performed, directly followed by injection of 2-[18F]fluoro-2-deoxy-D-glucose ([18F]FDG; 185 MBq) and a dynamic positron emission tomography (PET) acquisition, during which heart rate was monitored. After termination of the scan, blood pressure and heart rate were measured, the final blood sample was drawn, and indirect calorimetry was performed for 30 min.