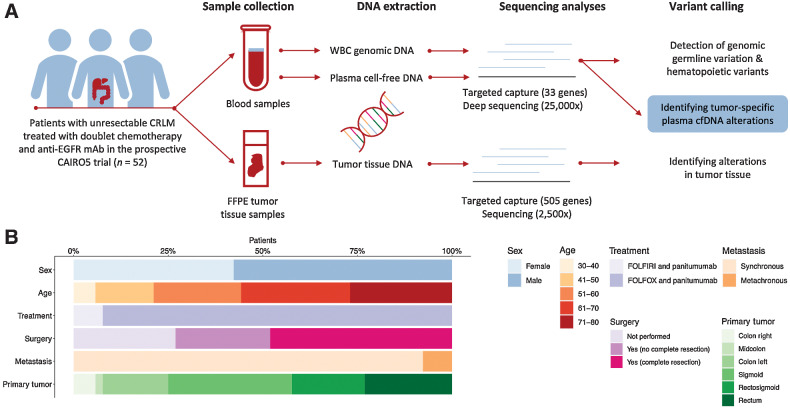
Figure 1.
Analyses of cfDNA in patients with CRLM. A, Schematic representation of the identification of tumor-specific somatic mutations. Blood and tumor tissue samples were collected from 52 patients with CRLM. WBC genomic DNA was isolated from the blood samples and used to remove germline and hematopoietic variants. Tumor tissue DNA was isolated and tumor alterations were identified. Tumor-specific somatic mutations were identified by subtracting the identified WBC hematopoietic and germline variants from the identified plasma cfDNA alterations. B, Baseline characteristics of the 52 patients with CRLM included. CRLM; colorectal liver metastases; EGFR, epidermal growth factor receptor; FFPE, formalin-fixed paraffin-embedded; FOLFIRI, folinic acid, fluorouracil, and irinotecan; FOLFOX, folinic acid, fluorouracil, and oxaliplatin; WBC, white blood cell.