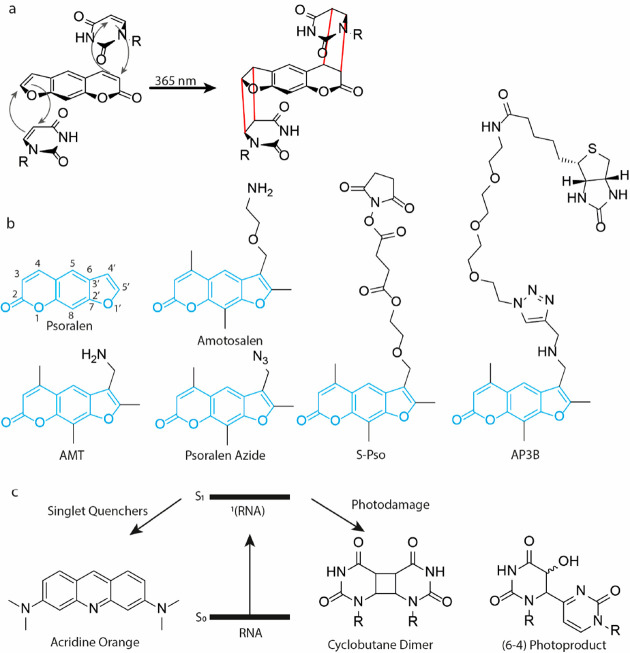
Figure 3.
Psoralen cross-linkers. (a) Cross-linking mechanism. Psoralen intercalates between opposing pyrimidines. UV exposure initiates a [2 + 2] photocycloaddition between the C5–C6 double bond of uracil or cytosine and the 3,4 and 4′,5′ double bond of psoralen to form a cyclobutane and cross-linking the pyrimidines. (b) Molecular structure of psoralen derivatives. (c) Singlet quenchers like acridine orange can protect RNA from photodamage.