Figure 4. GSDMD is dispensable for NLRP3‐mediated ASC oligomerisation downstream of caspase‐8.
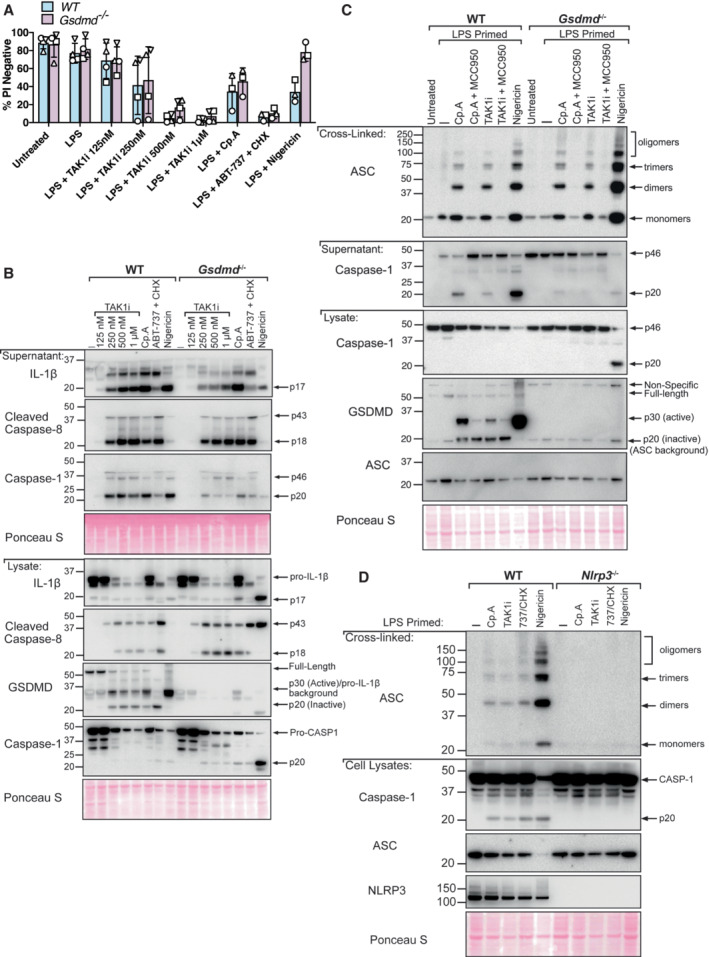
- BMDMs of the indicated genotypes were primed with 100 ng/ml of LPS for 3 h before treatment with 5Z‐7 oxozeaenol (TAK1i, 125 nM, 250 nM, 500 nM, and, 1 μM), Cp. A (1 μM) or ABT‐737 (1 μM) and CHX (20 μg/ml) for 6 h, or nigericin (10 μM) for 20 min. Cell viability was measured by propidium iodide (PI) uptake and flow cytometry and expressed as a percentage of PI‐negative (live) cells. Three to four independent experiments are shown (symbols), and error bars represent the mean ± SD.
- BMDMs of the indicated genotypes were primed with 100 ng/ml of LPS for 3 h before treatment with 5Z‐7 oxozeaenol (TAK1i, 125 nM, 250 nM, 500 nM, and, 1 μM), Cp. A (1 μM) or ABT‐737 (1 μM) and CHX (20 μg/ml) for 6 h, or nigericin (10 μM) for 45 min. Cell supernatants and total cell lysates were analysed by western blot. Ponceau staining depicts protein loading. One of three independent experiments.
- BMDMs of the indicated genotypes were seeded at a density of 2 × 106 cells per well and primed with LPS (100 ng/ml) for 3 h before treatment with Cp. A (1 μM) or 5Z‐7 oxozeaenol (TAK1i, 250 nM) with or without the NLRP3 inhibitor MCC950 (5 μM) for 6 h or were treated with nigericin (10 μM) for 45 min. Cell lysates and supernatants were analysed by western blot. Following freeze‐thawing of cells, the PBS‐insoluble fraction of the cell lysate was cross‐linked and assessed for ASC oligomerisation by western blot. Ponceau staining depicts protein loading. Data representative of three independent experiments.
- BMDMs of the indicated genotypes were seeded at a density of 2 × 106 cells per well and primed with 100 ng/ml LPS for 6 h before treatment with Cp. A (1 μM) or TAK1i (250 nM) for 6 h, ABT‐737 (1 μM) and CHX (20 μg/ml) for 3 h and Nigericin (10 μΜ) for 1 h. PBS insoluble fractions of cell lysates were cross‐linked to assess ASC oligomerisation and these, alongside cell lysates and supernatants, were analysed by western blot. Data represent two independent experiments.
Source data are available online for this figure.
