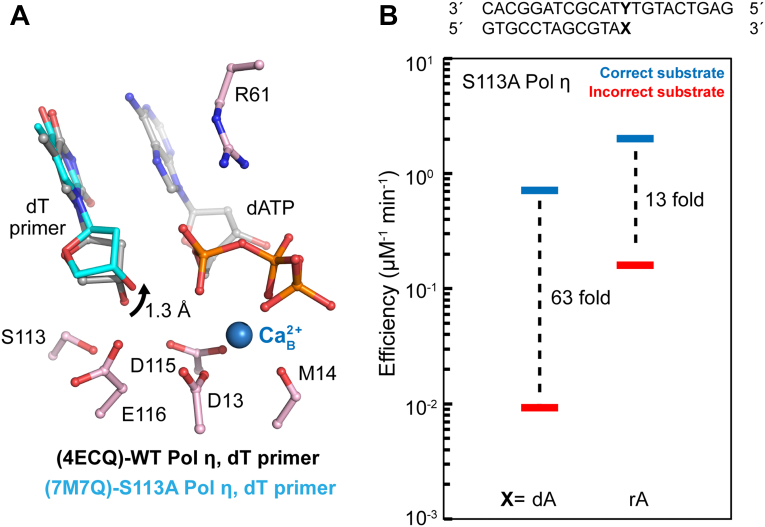
Figure 3.
Mechanisms of S113 A polymerase η catalysis and misincorporation. A, WT Pol η ground state with the dT primer terminus close to S113 (PDB ID 4ECQ). The primer 3′-OH is slightly too low or close to S113 to be in an aligned conformation. The angle of the primer 3′ oxygen, substrate phosphorus, and its bridging oxygen are at 160˚. dT primer terminus close to S113 (light blue) in the S113 A Pol η ground state (PDB ID 7M7Q). The dT primer terminus moves 1.3 A toward the misaligned conformation when an alanine is substituted in place of S113. B, S113 A Pol η DNA polymerase correct and incorrect substrate incorporation efficiency during dN or rN extension in the presence of Mg2+ based on steady-state kinetics. The fold-change between correct and incorrect substrate incorporation efficiencies represents a measurement of substrate discrimination. The bars represent the mean of triplicate measurements for the catalytic efficiencies (kcat/KM) for incorporation of dATP (blue) and dGTP (red) opposite dT. Data are generated from Table S2. dGTP, deoxyribose guanine triphosphate; dN, deoxynucleotide; Pol η, DNA polymerase η; rN, ribonucleotide; dT, deoxyribose thymine.