Abstract
Background:
Both activated by environmental odorants, there is a clear role for the intranasal trigeminal and olfactory nerves in smell function. Unfortunately, our ability to perceive odorants decreases with age or with injury, and limited interventions are available to treat smell loss.
Objective:
We investigated whether electrical stimulation of the trigeminal nerve via trigeminal nerve stimulation (TNS) or transcranial direct current stimulation (tDCS) modulates odor sensitivity in healthy individuals.
Methods:
We recruited 20 healthy adults (12 Female, mean age = 27) to participate in this three-visit, randomized, double-blind, sham-controlled trial. Participants were randomized to receive one of three stimulation modalities (TNS, tDCS, or sham) during each of their visits. Odor detection thresholds were obtained at baseline, immediately post-intervention, and 30-min post-intervention. Furthermore, participants were asked to complete a sustained attention task and mood assessments before odor detection testing.
Results:
Findings reveal a timeXcondition interaction for guaiacol (GUA) odorant detection thresholds (F (3.188, 60.57) = 3.833, P = 0.0125), but not phenyl ethyl alcohol (PEA) odorant thresholds. At 30-min post-stimulation, both active TNS and active tDCS showed significantly increased sensitivity to GUA compared to sham TNS (Sham TNS = −8.30% vs. Active TNS = 9.11%, mean difference 17.43%, 95% CI 5.674 to 29.18, p = 0.0044; Sham TNS = −8.30% vs. Active tDCS = 13.58%, mean difference 21.89%, 95% CI 10.47 to 33.32, p = 0.0004).
Conclusion:
TNS is a safe, simple, noninvasive method for boosting olfaction. Future studies should investigate the use of TNS on smell function across different stimulation parameters, odorants, and patient populations.
1. Introduction
Smell loss is a critical sensory deficit associated with everyday dangers such as the inability to smell spoiled or burning food, psychosocial problems including social isolation, relationship difficulties, anhedonia, and reduced quality of life [1–4], as well as neuropsychiatric disorders [5–11] and an increased risk of mortality [12,13]. Even before the COVID-19 pandemic, which saw a significant increase in smell dysfunction [14], smell loss was not uncommon in the general population. In fact, an epidemiological study indicated measurable smell dysfunction in 12.5% of Americans over the age of 40, and 32% over the age of 80 [15]. While future prevalence rates of persistent dysfunction are likely to be more widespread, a lack of widely accepted, evidence-based, interventions available to treat smell dysfunction exist, creating a large gap in healthcare that is likely to escalate due to COVID [16]. Although some interventions including surgery and anti-inflammatory medications that target the nasal passages [17,18] are available to treat obstructive smell loss (e.g. chronic rhinosinusitis [19], viral-related inflammation [20], polyps [21]), there are no established interventions for dysfunction due to other, non-obstructive, etiologies (e.g. age and COVID) [22].
Olfaction, or how the brain processes airborne chemical molecules known as odorants, is achieved through two overlapping, and functionally coupled cranial nerve systems, the intranasal trigeminal and olfactory neurocircuits [23,24]. These two cranial nerve systems are responsible for the discrete perception of specific odorant features. The intranasal trigeminal circuit relays information regarding the pungency or “feel” of the stimulus and whether it produces, for example, a tingling, cooling, burning, or painful sensation. The olfactory circuit relays information more traditionally considered as olfaction, odorant quality (e.g., type, hedonic valence, familiarity, etc.). Odorants are often classified according to their olfactory- or trigeminal-activation profiles or varying affinities to these two circuits. Most odorants, including the “smoke-like” guaiacol (GUA), which has strong trigeminal properties, activate both cranial nerve systems concurrently. However, there are odorants that possess relatively weaker trigeminal properties and are thus preferentially processed by the olfactory neurocircuit. For example, phenyl ethyl alcohol (PEA), a “rose-like” odorant commonly used in the clinical evaluation of olfactory function, is considered one of these “olfactory” odorants [23]. Although the olfactory and intranasal trigeminal circuits have different roles in the perception of odorants, they also work closely together, influencing the activity of one another [25–28]. This suggests that stimulation of the trigeminal circuit could potentially improve olfactory function in humans by reducing detection thresholds and thereby increasing sensitivity to odorants.
Non-invasive methods of cranial nerve stimulation, including trigeminal nerve stimulation (TNS), are emerging forms of “bottom-up” transcranial electrical stimulation techniques [29]. Specifically, TNS is most commonly delivered at low-level electrical current (<5 mA), through electrodes attached to the forehead targeting the supraorbital nerve within the ophthalmic dermatome region of the trigeminal nerve [30,31]. TNS is presumed to activate the trigeminal afferent sensory pathway – mediated by brainstem nuclei including the trigeminocervical complex (TCC), after which the signal propagates up to key central nervous targets including thalamus and insular cortex [32]. To date, TNS has shown promise in the treatment of numerous neuropsychiatric conditions [33] including depression [34], posttraumatic stress disorder [35], epilepsy [36] and migraine [37]. Additionally, there are three commercially available and FDA-cleared TNS Devices: 1) the Monarch eTNS system, which administers stimulation during sleep for the treatment of adolescent attention deficit hyperactivity disorder (ADHD) [38], 2) the Cefaly device for migraine, and 3) the Sparrow Therapy System for opioid withdrawal symptom relief [39]. Although TNS has shown recent potential as a neuromodulation technique for both laboratory and at-home applications, its effects on smell function have yet to be explored.
Given the coupled roles in the perception of odorants by the intranasal trigeminal and olfactory cranial nerve systems, it is plausible that specific interventions targeting trigeminal neurocircuitry may be a therapeutic target for smell loss. Moreover, due to its proven safety, relatively easy administration, and inexpensive cost, TNS has potential as a highly scalable intervention. Thus, this proof-of-concept, pilot study in healthy adults explored whether modulating the trigeminal nerve via TNS influenced detection thresholds for two different odorants. A three-visit, double-blind, crossover trial was utilized to compare the effects of TNS on odor sensitivity compared to sham-TNS and to an active-control stimulation known as transcranial direct current stimulation (tDCS). While tDCS has comparable methodology to TNS, it employs a different waveform and basic underlying mechanism which could impact the behavioral effects of this paradigm.
2. Materials and methods
2.1. Study overview
20 healthy adults (12F/8 M, mean age of 27 ± 8.1 years, range 22–58) were recruited into this double-blind, three-visit, randomized, sham-controlled, crossover pilot trial investigating the effects of trigeminal nerve stimulation (TNS) and transcranial direct current stimulation (tDCS) on odor detection sensitivity. This study was approved by the Medical University of South Carolina (MUSC) IRB and all research activities were conducted at the MUSC Department of Psychiatry. After participants gave informed written consent, they were asked to attend three experimental visits that differed only by stimulation condition (TNS, tDCS, SHAM TNS). At each of the three visits, baseline odor detection, self-reported sleepiness and mood ratings, and a sustained attention task was acquired, followed by one of three randomized stimulation conditions. Odor detection thresholds, self-report scales, and sustained attention tasks were again acquired immediately post-stimulation as well as 30 min post-stimulation (Fig. 1). The time between visits was at least 24 h apart, and all visits were conducted during normal business hours (9AM-5PM). Experimental visit times were variable between participants, however within participant visit time remained consistent over the three experimental visits. The primary outcome measure for this trial was the stimulation effects on odor sensitivity to two different chemical odorants (PEA and GUA), with secondary outcomes of mood and attentional effects.
Fig. 1. Study timeline.
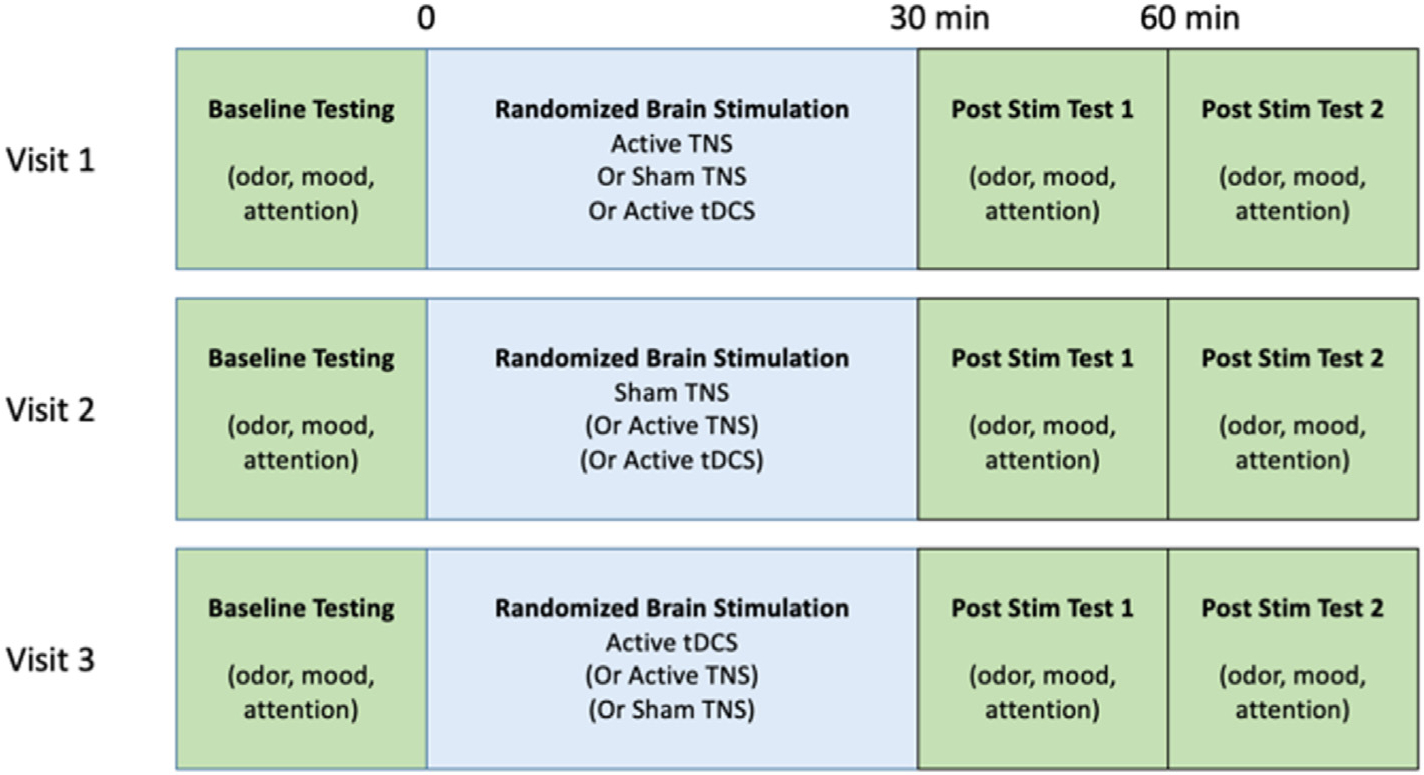
Experimental Timeline of the Study. We utilized a randomized, sham-controlled, prospective, crossover design during which participants returned to the laboratory for three discrete visits. Each visit was identical except for the condition of stimulation received.
2.2. Participant screening procedures
Recruitment ocurred between December 2019 through October 2020. All healthy, medication-free, participants were screened and excluded for history of COVID-19 infection, as well as all major medical and mental health conditions including drug use or dependence (except caffeine), trauma or damage to the nose or sinus conditions (e.g., impaired airflow, chronic rhinosinusitis, seasonal allergies), or olfactory function (e.g., current, or past loss or dysfunction). Furthermore, participants were only included if they attested to having a normal sense of smell and no history olfactory issues.
2.3. Olfactory detection threshold testing
Thresholds for both PEA and GUA were obtained with two versions of the Snap and Sniff ® Threshold Test (Sensonics International, Haddon Heights, NJ, USA). Each contained a series of wands with decreasing concentration of odorant (PEA or GUA) that ranged from the most intense (10−2) to the least intense (10−9) concentration. In a single staircase method with forced choices, a wand containing a given concentration of odorant was presented under the nose in rapid succession with an odorless wand. Study participants made a choice as to which wand had the stronger smell. Subsequent presentation of odorant (higher or lower concentration) against odorless was dependent on a correct or incorrect response for each trial. This method was repeated until seven reversals (up and down the staircase) were made. Detection threshold was determined by the mean of the last 4 reversals [40,41]. Each administration of the Snap and Sniff ® Threshold Test took approximately 5–10 min.
2.4. TNS and tDCS administration
Two forms of transcranial electrical stimulation were administered, trigeminal nerve stimulation (TNS) and transcranial direct current stimulation (tDCS). The administration of both forms of neurostimulation were identical with respect to the electrode attachment to the forehead but differed with respect to the waveform and hypothesized mechanism of action produced by the transcranial electrical stimulation systems (Fig. 2a). While TNS electrodes on the forehead target the supraorbital nerve within the ophthalmic dermatome region of the trigeminal nerve, tDCS electrodes attached to the forehead are suspected to activate both the trigeminal nerve, transcuatneously, as well as potentially deeper electrical current activation of the cortex (29). Irrespective of stimulation condition, all participants foreheads were first cleaned using an alcohol wipe. Next, 2in × 2in adhesive electrodes that utilized a stainless-steel knit cloth and hydrogel construction (Axelgaard Manufacturing Co., Ltd.) were attached to EEG Positions FP1 and FP2. These commercially available neurostimulation electrodes are designed with volume resistivity of < 1.5 kΩ-cm and do not induce cytotoxicity, irritation, or sensitization upon skin contact, and are very well tolerated by participants. A flexible athletic headband was placed over the electrodes to keep them firmly in place. Depending on condition, the electrodes were then connected into one of two different transcranial electrical stimulators described below.
Fig. 2. Setup of stimulation to target underlying trigeminal nerve anatomy.
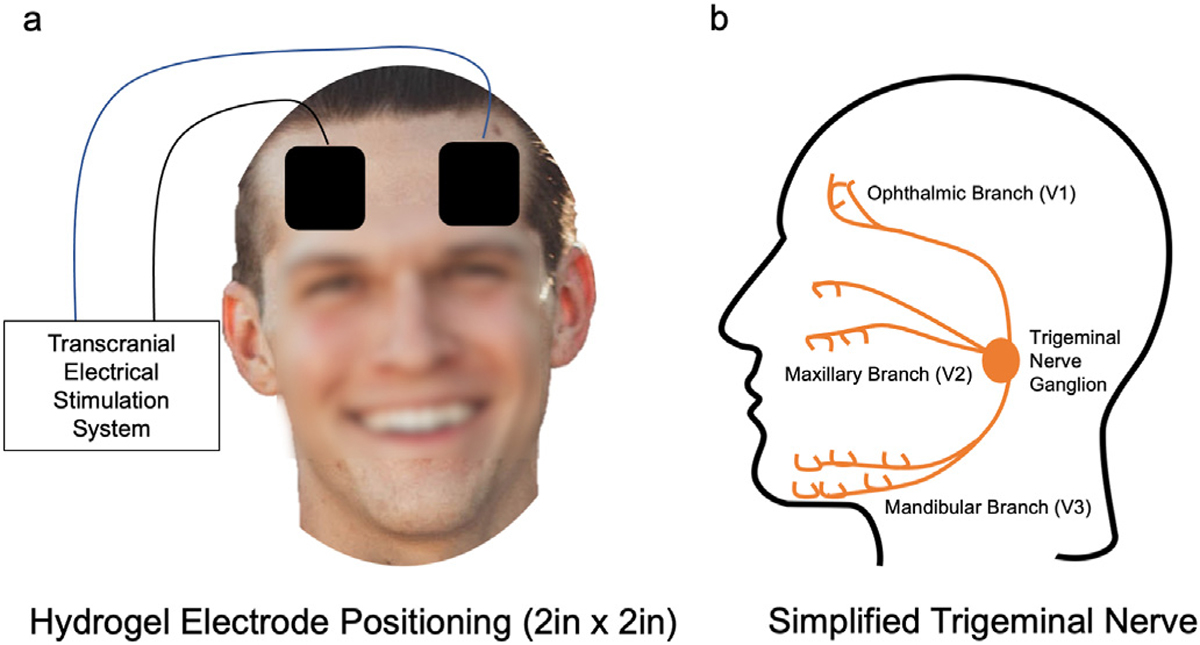
Overview of the electrode size and orientation for neurostimulation. Both tDCS and TNS utilized the same electrode position on the scalp (a), which allowed for stimulation of the ophthalmic branch of the trigeminal nerve. The electrical stimulator differed between tDCS and TNS. tDCS delivered a direct current stimulation, making the polarity important (anode on the right forehead, cathode on left). For TNS, the polarity placement was inconsequential due to the waveform. b) a simplified schema of the trigeminal nerve demonstrating three distinct branches and the stimulation target ophthalmic branch (V1).
For active and sham TNS, electrodes were plugged into an EMS 7500 digital TENS system (Roscoe Medical). Due to the bidirectional waveform, polarity of electrodes connection is inconsequential. TNS stimulation settings were based upon previous TNS studies [37,42] to include 120Hz, 30s ON, 30s OFF, stimulation administered for 30 min to the ophthalmic branch of the trigeminal nerve (Fig. 2b). This frequency is believed to engage the afferent trigeminal sensory pathway [38]. After placement of the forehead electrodes, we first conducted an initial perception and comfort procedure (ramping up to level 2) then turning off the system. After this initial comfort procedure, the device was turned back on set to either level 2 (active) or level 0 (sham) and programmed to start. Although the stimulation administrator was aware of the settings, these procedures were intended to keep the participant and the experimenter that determined the olfactory thresholds blinded by maintaining identical procedures.
For tDCS, electrodes were plugged into a Soterix 1 × 1 tDCS system (Soterix Medical). In tDCS, polarity of electrodes is important. The electrode placed over FP2 served as the anode (red), while the electrode placed over FP1 served as the cathode (black). Stimulation intensity was set to 1.5 mA administered for 30 min.
During all stimulation periods, participants were asked to sit in a relaxed position in a comfortable chair and watch a nature video on a 55” LED TV (https://www.youtube.com/watch?v=CnmLgezy3jc).
2.5. Blinding
Participants were not privileged to the form of stimulation they received each visit. Specifically, they were informed that they would receive “three different intensities of forehead stimulation”. A dedicated team member (EG) was solely responsible for the setup and administration of all stimulation methodology. Given the identical nature of electrode placement for all three stimulation conditions, this individual was primarily responsible for connecting electrodes into one of two different stimulation systems hidden behind the participant. This individual was not involved in any data analyses. A second team member (BC), who was not privileged to the stimulation conditions or systems, conducted all odor testing. A third team member (GHO), who was not involved in any data collection, unblinded and sorted the data for analysis upon completion of the study.
2.6. Self-reported sleepiness and mood scales
We utilized 100-mm Visual Analog Scales (VAS) to assess self-reported mood state. All participants completed VAS ratings regarding the extent to which they felt “angry”, “calm”, energized”, “happy”, “relaxed”, “restless”, and “tired” at baseline, post-stimulation, and again at 30-min post-stimulation. Statements for each rating used anchor points of 0 = ” not at all” to 100 = ” extremely”. Participants used a pen to mark the point on the line that best corresponded to how much they agreed with the statement (e.g., I feel relaxed). The VAS is a widely used, valid and reliable, means of rapidly quantifying subjective mood states [43].
The Stanford Sleepiness Scale (SSS) was utilized to assess participant sleepiness given the use of TNS during sleep in prior ADHD trials. The SSS is a simple 7-point rating scale developed by Hoddes and colleagues [44], and is the gold-standard for measuring subjective sleepiness. It is a validated and widely used tool in clinical trials. The SSS was not administered to the first four participants, due to the late incorporation of the metric, and thus only 16 of 20 participants completed this questionnaire.
2.7. Sustained attention to response test (SART)
Participants completed the computerized SART (Millisecond Software, LLC) at baseline, post-stimulation, and 30-min post-stimulation in the same quiet, experimental laboratory setting in which they received neurostimulation. The SART is a task that is designed to measure sustained attention and inhibitory control (go, no-go task) by requiring a motor response to frequent stimuli and a withheld motor response to a rare stimulus. The SART methodology utilized in this trial is described in detail by Jha and colleagues [45]. Reaction times of all key presses, including changes in errors of commission, errors of omission, and reaction times were logged in software and stored for analysis.
2.8. Statistical analysis
All participant data were analyzed, without any removal or dropout (i.e., n = 20), except for the analysis of the Stanford Sleepiness Scale (i.e., n = 16) due to the late incorporation of the metric. Repeated measures ANOVA with Geisser-Greenhouse correction were conducted using GraphPad Prism 9 to determine main effects of time, condition, and interactions for the overall effects of stimulation on detection thresholds of both PEA and GUA odorants, together and independently. Further odorant-specific analysis included ANOVAs with baseline-corrected percent change measures for odorants at the post-stimulation and 30-min post-stimulation timepoints with post-hoc pairwise Dunnett’s multiple comparison analysis to assess both active TNS and active tDCS effects to the sham TNS (control) condition. Similar methodology was utilized to assess the change scores from the Stanford Sleepiness Scale.
3. Results
3.1. Effects of stimulation on sensitivity to odorants
In one overall analysis, we investigated the effects on detection sensitivity thresholds for three timepoints (baseline, post-stim, and 30-min post) for GUA and PEA odorants combined, which revealed trending condition-by-time (F [4,76] = 2.04, p = 0.098, ) and odor-by-condition-by-time (F [4,76] = 2.46, p = 0.052, ) interactions suggesting that all brain stimulation conditions had an overall marginal effect on odor sensitivity detection thresholds, irrespective of odorant. These findings suggested that there may be effects specific to just one of the odorants.
An odorant specific repeated measures ANOVA indicated a timeXcondition effect only in the GUA detection threshold sensitivity (F (3.188, 60.57) = 3.833, P = 0.0125) (Fig. 3a) and no significant effects in the PEA odorant analysis (F (3.343, 63.52) = 1.121, P = 0.3502). Therefore, we explored the baseline corrected percent change of detection thresholds for GUA, which revealed a main effect of stimulation condition on sensitivity to GUA (F (1.795, 34.10) = 4.779, P = 0.0176). Further pairwise analysis of the condition-specific effects on GUA sensitivity using a Dunnett’s test of multiple comparisons investigating both active conditions independently to sham TNS revealed that there were no significant effects of either active stimulation condition compared to sham TNS at the post-stimulation time point (Mean difference change between Sham TNS vs. Active TNS: 13.22%, 95% CI: −8.810 to 35.24; Mean difference change between Sham TNS vs. Active tDCS: 9.718%, 95%CI: −6.855 to 26.29). However, at the 30min post-stimulation time point, both active TNS and active tDCS showed significantly increased sensitivity compared to sham TNS (Sham TNS = −8.30% vs. Active TNS = 9.11%, mean difference 17.43%, 95% CI 5.674 to 29.18, p = 0.0044: Sham TNS = −8.30% vs. Active tDCS = 13.58% mean difference 21.89%, 95% CI 10.47 to 33.32, p = 0.0004) (Fig. 3b). For reference, mean detection thresholds of the group level data have been included in Table 1.
Fig. 3. Effects of stimulation on GUA detection sensitivity thresholds.
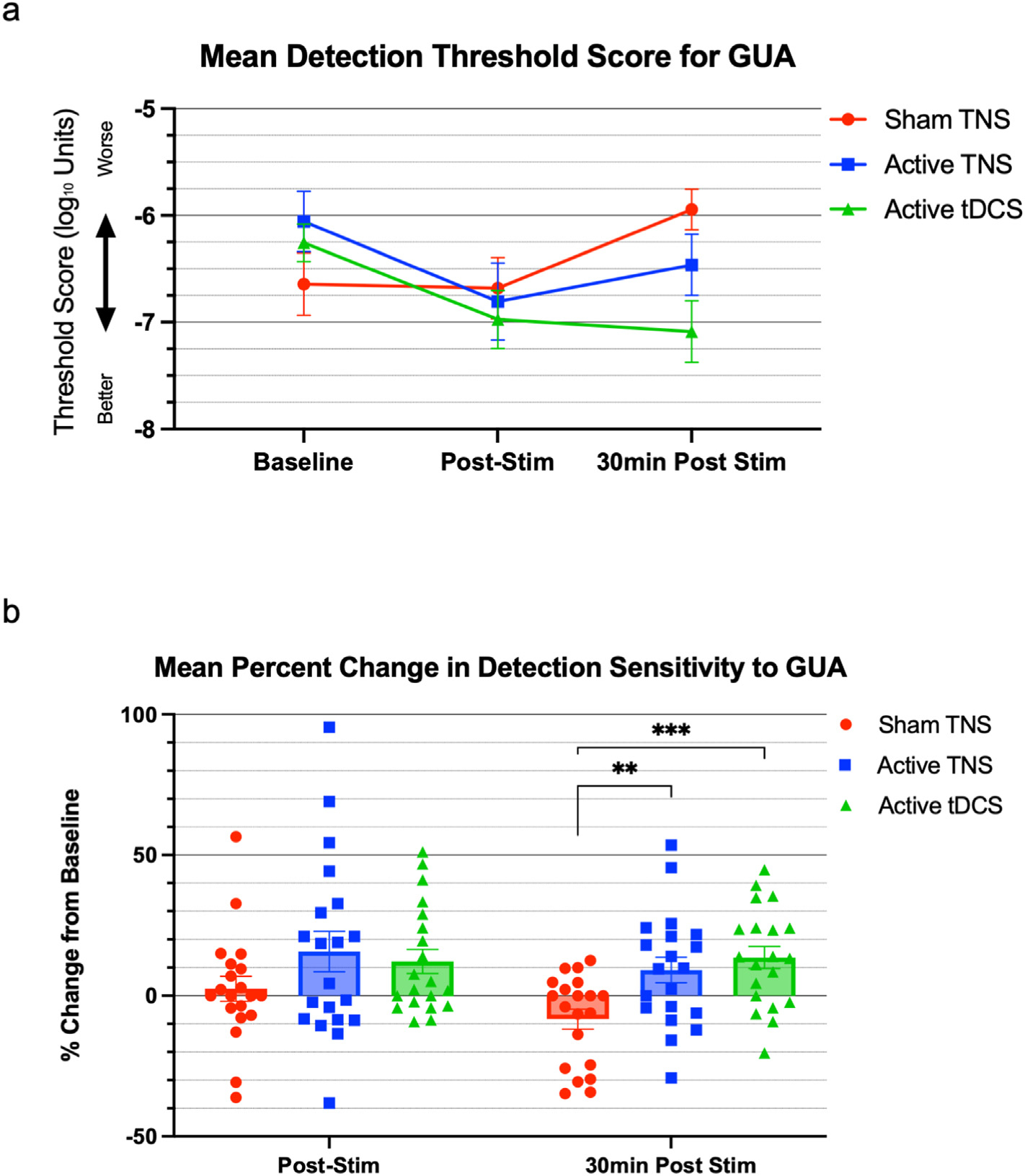
(a) Both active trigeminal nerve stimulation (TNS) and transcranial direct current stimulation (tDCS) induced reductions in GUA threshold score over time (lower mean odorant detection thresholds demonstrate improved smell detection) compared to baseline levels, and (b) significantly improve smell function at the 30-min post stimulation timepoint demonstrated by the mean percent change from baseline at the 30-min post stim time point compared to sham stimulation. Individual percent change data of all 20 participants is overlaid upon the mean bar chart. Error bars = SEM.
Table 1.
Mean and SD olfactory threshold levels for study timepoints and stimulation conditions.
| Odorant | Time | Sham TNS |
Active TNS |
Active tDCS |
|||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
|
| |||||||
| PEA | Baseline | −6.6063 | 1.695 | −6.3625 | 1.529 | −6.575 | 1.68 |
| Post-Stim | −7.575 | 1.116 | −6.4125 | 2.215 | −7.1875 | 1.738 | |
| 30min Post-Stim | −6.7063 | 1.465 | −6.375 | 1.981 | −6.8375 | 1.891 | |
| GUA | Baseline | −6.643 | 1.309 | −6.056 | 1.262 | −6.2563 | 0.785 |
| Post-Stim | −6.681 | 1.285 | −6.806 | 1.607 | −6.975 | 1.208 | |
| 30min Post-Stim | −5.943 | 0.844 | −6.462 | 1.278 | −7.0875 | 1.29 | |
3.2. Effects of stimulation on sleepiness
A repeated measures ANOVA of the percent change in sleepiness in 16 individuals revealed a significant main effect of condition (F (1.769, 26.53) = 5.141, P = 0.0157), indicating that stimulation condition impacts sleepiness. A further Dunnett’s test for multiple comparisons revealed that participants were significantly less sleepy after active, compared to sham, TNS stimulation (Sham TNS = 1.125 vs. Active TNS = 0.25, mean difference −0.875, 95% CI: −1.734 to −0.01617, p = 0.0458). This reduction of sleepiness was sustained at the 30-min follow up time point (Sham TNS = 1.187 vs. Active TNS = 0.125, mean difference. −1.063, 95% CI: −1.966 to −0.1588, p = 0.0216), There were no significant changes reported between the active tDCS and sham TNS conditions. (Fig. 4).
Fig. 4. Effects of stimulation on subjective sleepiness.
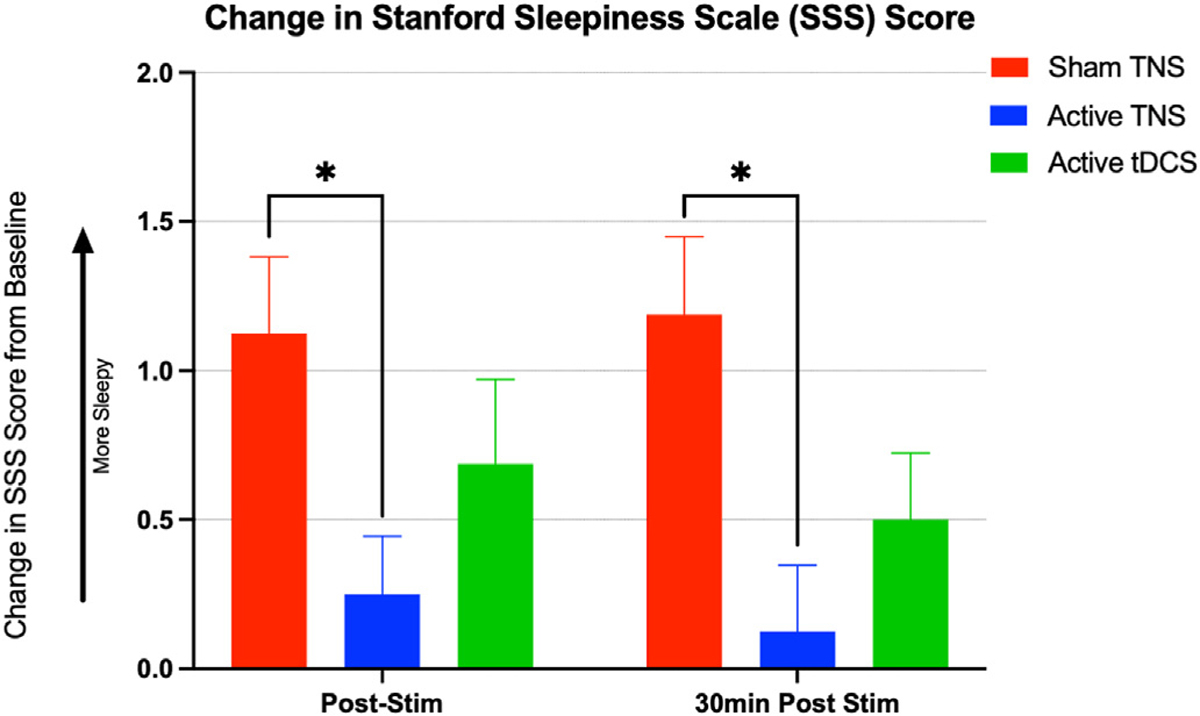
Mean change in self-reported sleepiness measured on a 7 point Likert scale using the Stanford Sleepiness Scale (SSS) immediately following and 30-min after trigeminal nerve stimulation (TNS), transcranial direct current stimulation (tDCS), and sham stimulation. Participants reported significantly less sleepiness after TNS, compared to sham stimulation, which suggests that TNS may have countered the sleepiness acquired during the relaxing study procedures. This effect was endured for at least 30-min post-stimulation. Error bars = SEM.
3.3. Effects of stimulation on mood
There were no significant condition or condition-by-time interactions revealed for any of the self-reported mood scales, indicating that neither tDCS nor TNS impacted subjective levels of anger, calmness, energy, happiness, relaxation, restlessness, or tiredness.
3.4. Effects of stimulation on sustained attention
We explored the effects of stimulation condition on sustained attention measures, including changes in errors of commission, errors of omission, and reaction times. All these analyses failed to reveal significant main effects of time and condition or time-by-condition interactions.
4. Discussion
Twenty healthy individuals were enrolled into a double-blind, randomized, sham-controlled trial exploring the use of TNS to improve smell. Our findings revealed that 30 min of neurostimulation delivered to the ophthalmic branch of the trigeminal nerve via trigeminal nerve stimulation (TNS) significantly improved sensitivity to guaiacol, an odorant that activates the intranasal trigeminal circuit, but not phenyl ethyl alcohol, an odorant with minimal action on this circuit. Immediately following stimulation, both active TNS and tDCS improved sensitivity to guaiacol (15.68% and 12.18% respectively) compared to sham TNS (2.5%) (Fig. 4b). Furthermore, both TNS and tDCS provided significant effects 30 min after stimulation, with sensitivity increases of 9.11% and 13.58%, respectively. Lastly, individuals receiving active TNS were significantly less sleepy after stimulation compared to the sham TNS group.
There are limited interventions for olfactory dysfunction, and our findings provide support for a brain-based intervention for improving detection. Currently, smell training (ST) is the only accepted intervention for age- or virus-related smell loss, the two most common and highly relevant forms of smell loss. ST, a system of olfactory exercise based on repeated exposures to familiar odorants, emerged recently as a promising approach for both ageand COVID-related dysfunction [46,47]. ST is not without limitations however, requiring a high level of patient compliance, long treatment course (12–16 weeks), no immediate effects, and ultimately low retention rates. TNS and tDCS, as described in our findings, may offer a safe and easy immediate boost to smell function.
Although the behavioral effects of both TNS and tDCS have been widely demonstrated in a variety of different neurological and psychiatric disorders, the underlying mechanism behind both these neuromodulation modalities has yet to be fully understood. Both TNS and tDCS fall under the category of transcranial electrical stimulation (TES) [48], however their current waveform is different. TNS pulses biphasic electrical current at 120Hz, while tDCS uses a monophasic square wave that is on for the duration of the intervention. This parametric difference has led to both nomenclature differences and also purported mechanistic differences. Historically, the field of neuromodulation has often ignored the cranial nerve activation profile of tDCS, opting for advanced electric field modelling of micro-changes in electrical fields at cortical and subcortical levels. However, here we compared both biphasic pulsed current and direct current to identical regions of the scalp, and our findings reveal both TNS and tDCS induce a significant and persistent modulation of guaiacol sensitivity, suggesting that improved detection may be mediated through direct activation of the trigeminal nerve. Alternatively, these neuromodulatory effects may be driven via activation of distal secondary olfactory cortex structures, such as the orbitofrontal cortex (OFC) which is highly associated with processing of odor learning and memory [49]. According to current modelling conducted by Manuel and colleagues [50], tDCS administered as described in this manuscript may induce electrical fields in smell related brain structures. It is not implausible that the effects of tDCS may also be due, in part, to modest activation of cortical structures that alter olfaction processing. It is important to note that regardless of the active stimulation modality, the post-stimulation timepoint may be confounded by a placebo effect in the sham condition which diminishes the effect size of both active conditions at the post-stimulation time point. This effect dissipates at the follow-up, similar to other sham neuromodulation interventions [51].
This double-blind pilot study was an attempt to determine whether TNS influenced the detection of odorants and was therefore conducted with healthy adults who were pre-screened and excluded for potential problems with sense of smell, other than normal aging. Given the longer-term goals of this research however, which are to develop TNS methodology to treat individuals with smell loss or other dysfunction including a distorted sense of smell, future studies will be needed to determine the efficacy as a treatment intervention for specific smell problems. As several different etiologies contribute to smell loss, these investigations will be necessary to determine whether TNS has utility in treating dysfunction due to various underlying pathologies. For example, it is not clear whether the effects of TNS translate to a disease population like COVID-related smell loss which impacts the sensitivity to a wide range of different odorants. Given that sensitivity to guaiacol, but not PEA, was significantly increased, our preliminary results suggest that TNS may improve sensitivity to some, but not other, odorants. Most odorants however, including those we naturally encounter, have some degree of trigeminal action. Thus, TNS may improve sensitivity to the majority of odorants perceived each day, and importantly to those that may signal potential danger (e.g., smoke). In addition to future studies to determine whether TNS reverses the impact of smell loss with different etiologies, other research will be required to determine how TNS produces its therapeutic effects, and whether its mechanism of action is in the periphery (e.g. receptor level), and/or involves central brain mechanisms.
During stimulation, regardless of active or sham, participants sat quietly watching a nature video for the entire 30 min, which may have been relaxing. Results for self-reported sleepiness indicated a significant increase for the sham-TNS (~16%), compared to active-TNS. While these results indicate possible sleepiness dampening effects for active stimulation, it is less likely that these effects contributed to the increased sensory function, as sleepiness was not reduced from baseline. Also, there were no effects of stimulation on sustained attention, suggesting that the brain mechanisms involved in TNS-induced increased sensitivity were not based in those that serve attention. Although our findings on odor detection are interesting, the anti-sleepiness, stimulant-like, effects of TNS have been established by several groups who are using these alerting, stimulant-like, effects to treat attention-deficit/hyperactivity disorder (ADHD). In the treatment of ADHD, users self-administer TNS to their forehead during sleep and, after four weeks of daily treatment, see significant and clinically meaningful reductions in ADHD symptoms. Mechanistically, these effects are likely mediated via the trigeminal nerve’s activation of the locus coeruleus, and subsequent noradrenaline release.
4.1. Limitations and future directions
A key limitation of our study is that our design utilized a single sham stimulation condition – TNS. TNS and tDCS, even when applied to identical targets, feel different and have variant electrical field activation profiles. Although our current design of using a sham TNS was imperceptible and served as a non-stimulation control, utilizing a standard tDCS sham (ramp up and ramp down) may serve as a more rigorous comparator to the active tDCS condition. Alternatively, an alternative stimulation location for TNS could have served as an active-sham comparator and may have provided information regarding the mechanism of action by stimulating a branch of the trigeminal that may not be involved in olfaction.
Furthermore, it is unclear whether these single-session effects are durable, as our last follow-up measurement was conducted only 30-min following the stimulation period. Typically, TNS is administered daily, for four to eight weeks. Therefore, we assume that increasing the number of sessions may increase the effects, and follow-up studies should explore this aspect of dosing.
Lastly, because olfaction was the primary outcome measure of this pilot trial, we did not control for factors that may have impacted secondary or exploratory outcomes. We did, however, mitigate those confounds by enrolling healthy, normosmic, non-smokers who were asked to maintain a regular routine during the course of their participation in this study. Although individual experimental visit times were variable between participants, within participants visit time remained consistent over the three experimental visits. Thus, we do not suspect non-conforming behaviors to confound these results.
5. Conclusions
This trial demonstrates the utility and efficacy of TNS and tDCS interventions to improve olfaction in healthy individuals. This application may be a promising approach to treating non-obstructive forms of smell loss, especially age- and COVID-related smell loss. Further studies should explore this approach in a disease population, including investigating the dosing parameters and administering this therapy in the at-home setting.
Footnotes
Declaration of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Bashar W. Badran: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition. Elise M. Gruber: Methodology, Validation, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Georgia H. O’Leary: Methodology, Validation, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Chris W. Austelle: Methodology, Validation, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Sarah M. Huffman: Methodology, Validation, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Alex T. Kahn: Methodology, Validation, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Lisa M. McTeague: Conceptualization, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition. Thomas W. Uhde: Conceptualization, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition. Bernadette M. Cortese: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
References
- [1].Keller A, Malaspina D. Hidden consequences of olfactory dysfunction: a patient report series. BMC Ear Nose Throat Disord 2013;13(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bensafi M, Rouby C. Individual differences in odor imaging ability reflect differences in olfactory and emotional perception. Chem Senses 2007;32(3):237–44. [DOI] [PubMed] [Google Scholar]
- [3].Zou LQ, Geng FL, Liu WH, Wei XH, Jiang XQ, Wang Y, et al. The neural basis of olfactory function and its relationship with anhedonia in individuals with schizotypy: an exploratory study. Psychiatr Res 2015;234(2):202–7. [DOI] [PubMed] [Google Scholar]
- [4].Philpott CM, Boak D. The impact of olfactory disorders in the United Kingdom. Chem Senses 2014;39(8):711–8. [DOI] [PubMed] [Google Scholar]
- [5].Cortese BM, Leslie K, Uhde TW. Differential odor sensitivity in PTSD: implications for treatment and future research. J Affect Disord 2015;179:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Croy I, Symmank A, Schellong J, Hummel C, Gerber J, Joraschky P, et al. Olfaction as a marker for depression in humans. J Affect Disord 2014;160:80–6. [DOI] [PubMed] [Google Scholar]
- [7].Moberg PJ, Kamath V, Marchetto DM, Calkins ME, Doty RL, Hahn CG, et al. Meta-analysis of olfactory function in schizophrenia, first-degree family members, and youths at-risk for psychosis. Schizophr Bull 2014;40(1):50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Atanasova B, Graux J, El Hage W, Hommet C, Camus V, Belzung C. Olfaction: a potential cognitive marker of psychiatric disorders. Neurosci Biobehav Rev 2008;32(7):1315–25. [DOI] [PubMed] [Google Scholar]
- [9].Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, Marder K, et al. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer’s disease at follow-up. Am J Psychiatr 2000;157(9):1399–405. [DOI] [PubMed] [Google Scholar]
- [10].Ross GW, Petrovitch H, Abbott RD, Tanner CM, Popper J, Masaki K, et al. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol 2008;63(2):167–73. [DOI] [PubMed] [Google Scholar]
- [11].Stiasny-Kolster K, Clever SC, Moller JC, Oertel WH, Mayer G. Olfactory dysfunction in patients with narcolepsy with and without REM sleep behaviour disorder. Brain 2007;130(Pt 2):442–9. [DOI] [PubMed] [Google Scholar]
- [12].Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One 2014;9(10):e107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gopinath B, Sue CM, Kifley A, Mitchell P. The association between olfactory impairment and total mortality in older adults. J Gerontol A Biol Sci Med Sci 2012;67(2):204–9. [DOI] [PubMed] [Google Scholar]
- [14].Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngol : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery 2020;277(8):2251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rawal S, Hoffman HJ, Bainbridge KE, Huedo-Medina TB, Duffy VB. Prevalence and risk factors of self-reported smell and taste alterations: results from the 2011–2012 US national health and nutrition examination survey (NHANES). Chem Senses 2016;41(1):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Doty RL, Kamath V. The influences of age on olfaction: a review. Front Psychol 2014;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rudmik L, Schlosser RJ, Smith TL, Soler ZM. Impact of topical nasal steroid therapy on symptoms of nasal polyposis: a meta-analysis. Laryngoscope 2012;122(7):1431–7. [DOI] [PubMed] [Google Scholar]
- [18].Schlosser RJ, Soler ZM. Evidence-based treatment of chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy 2013;27(6):461–6. [DOI] [PubMed] [Google Scholar]
- [19].Simola M, Malmberg H. Sense of smell in allergic and nonallergic rhinitis. Allergy 1998;53(2):190–4. [DOI] [PubMed] [Google Scholar]
- [20].Klopfenstein T, Kadiane-Oussou NJ, Toko L, Royer PY, Lepiller Q, Gendrin V, et al. Features of anosmia in COVID-19. Med Maladies Infect 2020;50(5):436–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Doty RL, Mishra A. Olfaction and its alteration by nasal obstruction, rhinitis, and rhinosinusitis. Laryngoscope 2001;111(3):409–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schiffman SS. Taste and smell losses in normal aging and disease. JAMA 1997;278(16):1357–62. [PubMed] [Google Scholar]
- [23].Doty RL, Brugger WE, Jurs PC, Orndorff MA, Snyder PJ, Lowry LD. Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol Behav 1978;20(2):175–85. [DOI] [PubMed] [Google Scholar]
- [24].Doty RL, McKeown DA, Lee WW, Shaman P. A study of the test-retest reliability of ten olfactory tests. Chem Senses 1995;20(6):645–56. [DOI] [PubMed] [Google Scholar]
- [25].Jacquot L, Monnin J, Brand G. Influence of nasal trigeminal stimuli on olfactory sensitivity. C R Biol 2004;327(4):305–11. [DOI] [PubMed] [Google Scholar]
- [26].Tremblay C, Frasnelli J. Olfactory and trigeminal systems interact in the periphery. Chem Senses 2018;43(8):611–6. [DOI] [PubMed] [Google Scholar]
- [27].Boucher Y, Simons CT, Faurion A, Azerad J, Carstens E. Trigeminal modulation of gustatory neurons in the nucleus of the solitary tract. Brain Res 2003;973(2):265–74. [DOI] [PubMed] [Google Scholar]
- [28].Cain WS, Murphy CL. Interaction between chemoreceptive modalities of odour and irritation. Nature 1980;284(5753):255–7. [DOI] [PubMed] [Google Scholar]
- [29].Adair D, Truong D, Esmaeilpour Z, Gebodh N, Borges H, Ho L, et al. Electrical stimulation of cranial nerves in cognition and disease. Brain Stimul 2020;13(3):717–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].DeGiorgio CM, Shewmon A, Murray D, Whitehurst T. Pilot study of trigeminal nerve stimulation (TNS) for epilepsy: a proof-of-concept trial. Epilepsia 2006;47(7):1213–5. [DOI] [PubMed] [Google Scholar]
- [31].Kamel HA, Toland J. Trigeminal nerve anatomy: illustrated using examples of abnormalities. AJR Am J Roentgenol 2001;176(1):247–51. [DOI] [PubMed] [Google Scholar]
- [32].Simpson KL, Altman DW, Wang L, Kirifides ML, Lin RC, Waterhouse BD. Lateralization and functional organization of the locus coeruleus projection to the trigeminal somatosensory pathway in rat. J Comp Neurol 1997;385(1):135–47. [PubMed] [Google Scholar]
- [33].Shiozawa P, Silva ME, Carvalho TC, Cordeiro Q, Brunoni AR, Fregni F. Transcutaneous vagus and trigeminal nerve stimulation for neuropsychiatric disorders: a systematic review. Arq Neuropsiquiatr 2014;72(7):542–7. [DOI] [PubMed] [Google Scholar]
- [34].Generoso MB, Taiar IT, Garrocini LP, Bernardon R, Cordeiro Q, Uchida RR, et al. Effect of a 10-day transcutaneous trigeminal nerve stimulation (TNS) protocol for depression amelioration: a randomized, double blind, and sham-controlled phase II clinical trial. Epilepsy Behav 2019;95:39–42. [DOI] [PubMed] [Google Scholar]
- [35].Cook IA, Abrams M, Leuchter AF. Trigeminal nerve stimulation for comorbid posttraumatic stress disorder and major depressive disorder. Neuromodulation 2016;19(3):299–305. [DOI] [PubMed] [Google Scholar]
- [36].Gil-Lopez F, Boget T, Manzanares I, Donaire A, Conde-Blanco E, Bailles E, et al. External trigeminal nerve stimulation for drug resistant epilepsy: a randomized controlled trial. Brain Stimul 2020;13(5):1245–53. [DOI] [PubMed] [Google Scholar]
- [37].Chou DE, Shnayderman Yugrakh M, Winegarner D, Rowe V, Kuruvilla D, Schoenen J. Acute migraine therapy with external trigeminal neurostimulation (ACME): a randomized controlled trial. Cephalalgia 2019;39(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McGough JJ, Sturm A, Cowen J, Tung K, Salgari GC, Leuchter AF, et al. Double-blind, sham-controlled, pilot study of trigeminal nerve stimulation for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2019;58(4):403–411 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jenkins DD, Khodaparast N, O’Leary GH, Washburn SN, Covalin A, Badran BW. Transcutaneous auricular neurostimulation (tAN): a novel adjuvant treatment in neonatal opioid withdrawal syndrome. Front Hum Neurosci 2021;15(110). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pierce JD Jr, Doty RL, Amoore JE. Analysis of position of trial sequence and type of diluent on the detection threshold for phenyl ethyl alcohol using a single staircase method. Percept Mot Skills 1996;82(2):451–8. [DOI] [PubMed] [Google Scholar]
- [41].Doty RL. The smell threshold test administration manual. second ed. Haddon Heights, NJ: Sensonics, Inc; 2009. [Google Scholar]
- [42].McGough JJ, Sturm A, Cowen J, Tung K, Salgari GC, Leuchter AF, et al. Double-blind, sham-controlled, pilot study of trigeminal nerve stimulation for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatr 2019;58(4):403–11. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ahearn EP. The use of visual analog scales in mood disorders: a critical review. J Psychiatr Res 1997;31(5):569–79. [DOI] [PubMed] [Google Scholar]
- [44].Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology 1973;10(4):431–6. [DOI] [PubMed] [Google Scholar]
- [45].Jha AP, Morrison AB, Dainer-Best J, Parker S, Rostrup N, Stanley EA. Minds “at attention”: mindfulness training curbs attentional lapses in military cohorts. PLoS One 2015;10(2):e0116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sorokowska A, Drechsler E, Karwowski M, Hummel T. Effects of olfactory training: a meta-analysis. Rhinology 2017;55(1):17–26. [DOI] [PubMed] [Google Scholar]
- [47].Addison AB, Wong B, Ahmed T, Macchi A, Konstantinidis I, Huart C, et al. Clinical Olfactory Working Group consensus statement on the treatment of postinfectious olfactory dysfunction. J Allergy Clin Immunol 2021;147(5):1704–19. [DOI] [PubMed] [Google Scholar]
- [48].Bikson M, Esmaeilpour Z, Adair D, Kronberg G, Tyler WJ, Antal A, et al. Transcranial electrical stimulation nomenclature. Brain Stimul 2019;12(6):1349–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Saive AL, Royet JP, Plailly J. A review on the neural bases of episodic odor memory: from laboratory-based to autobiographical approaches. Front Behav Neurosci 2014;8:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Manuel AL, David AW, Bikson M, Schnider A. Frontal tDCS modulates orbitofrontal reality filtering. Neuroscience 2014;265:21–7. [DOI] [PubMed] [Google Scholar]
- [51].Burke MJ, Kaptchuk TJ, Pascual-Leone A. Challenges of differential placebo effects in contemporary medicine: the example of brain stimulation. Ann Neurol 2019;85(1):12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


