Figure 1. SPINK6 constrains proteolytic activity of trypsin, and trypsin‐mediated HA cleavage and viral growth.
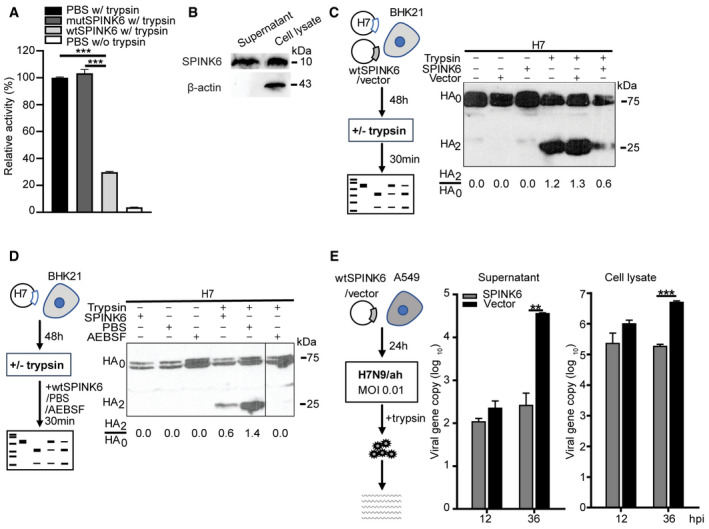
- TPCK trypsin is premixed with wtSPINK6 protein or mutSPINK6 protein or PBS in triplicate, incubated with a fluorogenic substrate for 30 min, and then applied to fluorescence assay. Data are presented as mean and SD in a representative experiment performed three times. The fluorescence intensity of TPCK trypsin/PBS mixture is arbitrarily set as 1. ***P < 0.001.
- At 48 h post‐transfection of wtSPINK6 plasmid in 293T cells, cell lysate and concentrated supernatant (50×) were applied to the detection of SPINK6 protein and β‐actin by WB.
- At 48 h after co‐transfection of H7 plasmid with wtSPINK6 plasmid or blank vector, the transfectants were incubated with or without TPCK trypsin for 30 min, and then applied to examine HA cleavage by WB. Intensities of HA2 and HA0 bands are quantified with ImageJ. HA2/HA0 ratio of each sample is shown at the bottom.
- At 48 h after transfection of H7 plasmid, the transfectants were incubated with TPCK trypsin in the presence of recombinant wtSPINK6 protein or PBS or a protease inhibitor AEBSF for 30 min and then applied to examine HA cleavage.
- At 24 h after transfection of wtSPINK6 plasmid or blank vector in triplicate, A549 cells were inoculated with H7N9/ah virus. At the indicated hours post‐infection (hpi), culture media (supernatant) and cell lysate were harvested for viral load detection. Data are presented as mean and SD in a representative experiment performed three times. **P < 0.01; ***P < 0.001. Student’s t‐test.
Source data are available online for this figure.
