Abstract
Purpose of review
The current review aims to present the latest scientific updates on the role of Sortilin in the pathophysiology of hypertension.
Recent findings
The main focus of this systematic overview is on the functional contribution of Sortilin to the pathogenesis of hypertension. Sortilin is a glycoprotein mostly known for its actions as a trafficking molecule directing proteins to specific secretory or endocytic compartments of the cell. Emerging evidence indicates that Sortilin is associated with pathological conditions, including inflammation, arteriosclerosis, dyslipidemia, insulin resistance, and vascular calcification. Most recently, Sortilin has been shown to finely control endothelial function and to drive hypertension by modulating sphingolipid/ceramide homeostasis and by triggering oxidative stress.
Summary
The latest findings linking Sortilin and hypertension that are herein discussed can inspire novel areas of research which could eventually lead to the discovery of new therapeutic strategies in cardiovascular medicine.
Keywords: ceramide, endothelium, hypertension, oxidative stress, sphingolipids
INTRODUCTION
Hypertension is one of the leading causes of death worldwide, and in the last decade a higher incidence has been documented, especially in the adult population. In general, hypertension does not cause any peculiar symptom, but in the long run it becomes a serious risk factor for other major conditions including stroke, coronary heart disease, heart failure, loss of vision, kidney disease, and dementia [1,2].
Hypertension is a complex polygenic disease, in which several genes, or a combination of these, can affect blood pressure [3-5]. To date, more than 25 rare mutations and 120 individual polymorphisms contributing to blood pressure have been recognized [3,6]. Moreover, there are several risk factors that can affect blood pressure, including excessive alcohol consumption, reduced physical activity and increased body mass index, and a diet with excessive sodium intake and/or insufficient intake of potassium [5,7,8]. All these factors alone or in combination cover a large percentage of triggers inducing hypertension.
One of the issues with hypertension is that being a highly heterogeneous disorder, the association of most biomarkers with the risk of hypertension has often been modest, thus being not easily applicable to a proper clinical use [9-17]; therefore, new research strategies are being developed, shifting the attention to other potential biomarkers, which may be soon become novel therapeutic targets. Sortilin represents one of the best examples in this sense.
SORTILIN
Sortilin is a single-pass type 1 transmembrane glycoprotein of approximately 95 kDa encoded by the SORT1 gene (also known as Glycoprotein 95, Gp95, or Neurotensin Receptor 3, NTR3) located on chromosome 1 in humans [18]. Sortilin belongs to the family of vacuolar protein domain receptors 10 (VPS10P), which includes both the Sortilin-related receptor 1 (SORL1) and the Sortilin Related VPS10 Domain Containing Receptor 1–3 (sorCS1–3).
Sortilin consists of a short cytoplasmic tail, a single transmembrane helix, and a large extracellular domain VPS10P. The protein acts as a receptor, co-receptor, and trafficking molecule [19,20■■,21,22,23■■].
Sortilin is synthesized as a precursor that includes in its luminal N-terminus a 44-residue-long pro-peptide, called Spadin or Sort-pro [24], which plays a fundamental role in facilitating transport of the pro-receptor through the biosynthetic pathway, until it is cleaved off in the trans-Golgi network by protein convertases [25,26]. In addition to being localized in intracellular compartments, including endoplasmic reticulum and Golgi, Sortilin is also expressed at the cell surface where it acts as an endocytotic receptor for various extracellular ligands. Sortilin is cleaved around the base of its luminal side by the Ca2+-regulated transmembrane sheddase ADAM10 (A Disintegrin And Metalloproteinase Domain-Containing Protein 10) [27], and the ectodomain is released as a soluble form into the extracellular space.
At the cellular level, Sortilin is involved in the intracellular transport between endoplasmic reticulum (ER), Golgi apparatus, lysosomes, and plasma membrane, regulating multiple biological processes including glucose and lipid metabolism, the trafficking of extracellular vesicles, as well as cell development and cell death [22,28,29]. Sortilin binds a variety of both circulating and transmembrane targets including cytokines, enzymes, peptides, and growth factors [24,25]. It is abundant in the central nervous system; in fact it was initially identified in the brain tissue and has been extensively studied in the pathobiology of neurological disorders [30-33]. Subsequently, Sortilin has been associated with other pathological conditions, including inflammation, arteriosclerosis, dyslipidemia, insulin resistance, vascular calcification, immune disorders, and cancer [23■■,34-38].
SORTILIN AND HYPERTENSION
In the last few years Sortilin has become the topic of a number of scientific studies, embodying a leading role in cardiovascular and metabolic diseases, shaping its potential functional contribution both as a reliable biomarker and as a potential therapeutic target.
Circulating Sortilin levels have been found to be associated with a higher risk of arteriosclerosis, major adverse cardiac and cerebrovascular events (MACCE), coronary heart disease, and peripheral arterial disease (PAD) [39,40,41,42■,43-50].
Sortilin has recently been proven to have a direct role in vascular function, specifically promoting endothelial dysfunction and hypertension [18]. Specifically, in a recent study, Di Pietro et al. [51■■] explored the role of Sortilin in hypertension. Experimental assays carried out in mice revealed that chronic and acute administration of Sortilin caused an increase in blood pressure.
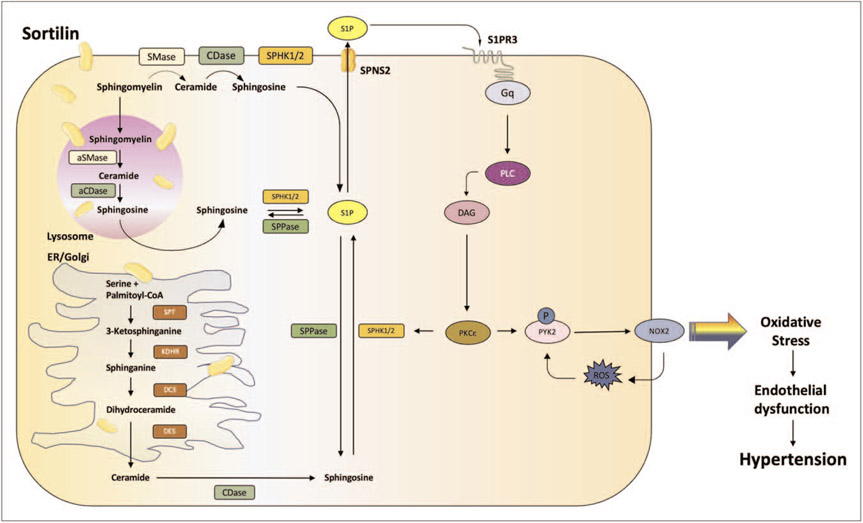
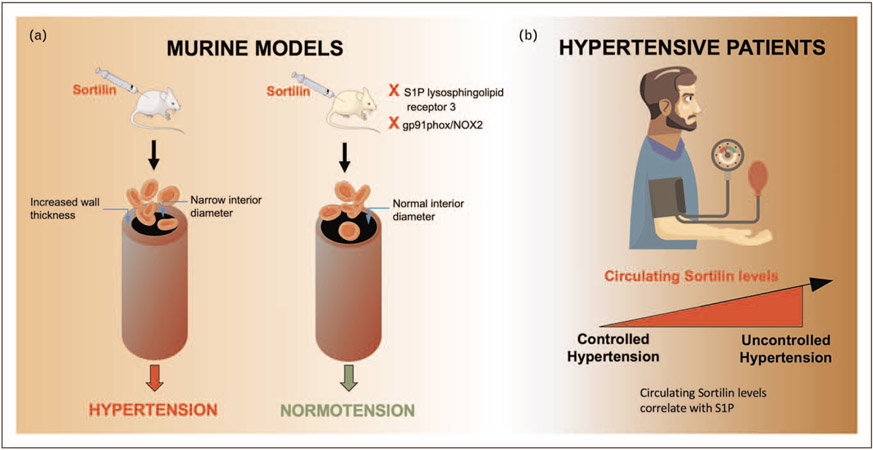
Sortilin alters the sphingolipid/ceramide homeostasis [18], initiating a signaling cascade (Fig. 1) that, from sphingosine-1-phosphate (S1P) leads to the augmented generation of reactive oxygen species (ROS) through the activation of the NADPH oxidase 2 (NOX2) isoform. Indeed, in mice without S1P lysosphingolipid receptor 3 (S1P3) or gp91phox/NOX2, Sortilin did not induce hypertension or vascular dysfunction (Fig. 2a), suggesting that these pathways are implied in the pathobiology of Sortilin-induced high blood pressure. In addition, using a pharmacological inhibitor (i.e. S1P3 TY52156), only an hour after the administration of Sortilin, it was possible to avoid the harmful effect of Sortilin and blood pressure in mice was lowered to normal values [51■■].
FIGURE 1.
Main signaling pathways mediated by Sortilin in the endothelial cell. aCDase, acid ceramidase; aSMase, acid sphingomyelinase; DAG, diacylglycerol; DCS, dihydroceramide synthase; DES, dihydroceramide desaturase; ER, endoplasmic reticulum; Gq, subunit q of G protein alpha; KDHR, 3-ketodihydrosphingosine reductase; NOX2, NADPH oxidase 2; PI3K, phosphatidylinositol 4,5-bisphosphate 3-kinase; PKCε, protein kinase C epsilon; PLC, phospholipase C; PYK2, protein tyrosine kinase 2 beta; ROS, reactive oxygen species; S1P, sphingosine 1 phosphate; S1PR3, type 3 sphingosine 1 phosphate receptor; SMase, sphingomyelinase; SPHK, sphingosine kinase; SPNS2, sphingolipid transporter 2; SPPase, S1P phosphatase; SPT, serine palmitoyltransferase.
FIGURE 2.
Preclinical and clinical investigations showing that Sortilin drives arterial hypertension. (a) Sortilin induces hypertension through a mechanism that involves sphingosine-1-phosphate (S1P) lysosphingolipid receptor 3 and/or gp91phox/NOX2. (b) Circulating Sortilin levels are greater in hypertensive patients with noncontrolled hypertension and correlate with S1P levels.
Strikingly, in experimental studies conducted in humans, circulating Sortilin levels were shown to be higher in hypertensive subjects with impaired endothelial function than in normotensive subjects [51■■]. Of note, these findings have been later confirmed by an independent investigation [52], which has shown that elevated Sortilin levels in the plasma are significantly associated with an increased risk of essential hypertension and subclinical carotid atherosclerosis in hypertensive patients (Fig. 2b).
As mentioned above, Sortilin has been associated with the presence of PAD, and hypertension is known to be one of the main risk factors for the development and progression of this disease [53]. The findings obtained by Di Pietro and coworkers were confirmed in hypertensive individuals with no previous symptoms or diagnosis of PAD, to rule out a possible influence of this comorbidity, confirming that Sortilin is involved in the pathogenesis of hypertension independently of other associated disorders.
In addition, hypertension is strongly connected with oxidative stress and endothelial dysfunction [18,54]. In fact, Sortilin has been shown to induce an over-production of ROS through the activation of NOX2 and to trigger endothelial dysfunction in murine mesenteric arteries [18].
These results support the theory that the activation of the S1P/NOX2 signaling axis induced by Sortilin and the subsequent impairment of endothelial-dependent dilation are involved in the increased blood pressure evoked by Sortilin.
Sortilin has been also linked to insulin resistance in type 2 diabetes mellitus and dyslipidemia, an association that has been suggested to be attributable to an altered hepatic metabolism of apolipoprotein B-100 (apoB-100) [55,56]. Genome-wide association studies have identified a locus on 1p13 as a risk locus for dyslipidemia and myocardial infarction, and a common noncoding polymorphism at the 1p13 locus, rs12740374, has been shown to alter the expression of the SORT1 gene [57■]. Actually, several single nucleotide polymorphisms (SNPs), that are coupled to an increased hepatic-specific SORT1 expression have been associated with reduced levels of low-density lipoprotein cholesterol (LDL-C) and apoB-100 [58,59]. A recent report demonstrated that these SORT1 SNPs were more protective against the risk of cardiovascular disease in diabetic patients compared to subjects without diabetes [60]. According to the latest studies, this protection could be attributable to a decrease in very-low-density lipoprotein (VLDL) production leading to reduced apoB containing lipoproteins in carriers of the SORT1 variants [61■]. Furthermore, Sortilin augments secretion of PCSK9 (proprotein convertase subtilisin/kexin type 9 serine protease), which binds the LDL receptor resulting in its endocytosis [62], thereby increasing the risk of atherosclerosis [55,63,64].
Sortilin is also functionally implied in the regulation of vascular calcification. Indeed, it has been shown to be upregulated during osteoblastic differentiation of mesenchymal stem cells and to promote extracellular matrix mineralization [65] and high circulating Sortilin levels have been associated with carotid calcification and severe carotid plaque score [66]. In a series of papers, Claudia Goettsch et al. [20■■,39,67] have elegantly demonstrated the mechanistic role of smooth muscle cell (SMC)-derived Sortilin in promoting vascular calcification mainly via its trafficking function of tissue non-specific alkaline phosphatase (TNAP) to extracellular vesicles triggering a high mineralization competence in the extracellular milieu; intriguingly, the injection of an adeno-associated virus encoding for PCSK9 to Sortilin-deficient mice led to a significantly reduced aortic calcification (by 46.3%) compared to littermate controls. Posttranslational modifications of Sortilin, including its carbamylation [42■] and its phosphorylation have been also associated with the calcification process [20■■].
CONCLUSION
Hypertension is a major widespread problem worldwide; moreover, in recent years its incidence has increased, and it is not excluded that there will be a further increase in the next decade [68]. Although today several pharmaceutical strategies are available, the percentage of successful treatment and control of hypertension is still considerably low, and therefore continues to be a nontrivial problem for public health. In addition, hypertension generally has no specific symptoms other than those attributable to its complications, and it is precisely for this reason that it is often called ‘silent killer’, explaining why finding a dependable way to foresee its actual manifestation, as for example a biomarker, is certainly very important for prevention. Sortilin therefore plays a very important role for this pathological condition and since high circulating levels of Sortilin have been detected in hypertensive patients, this protein could act as a powerful biomarker for the clinical management of hypertension.
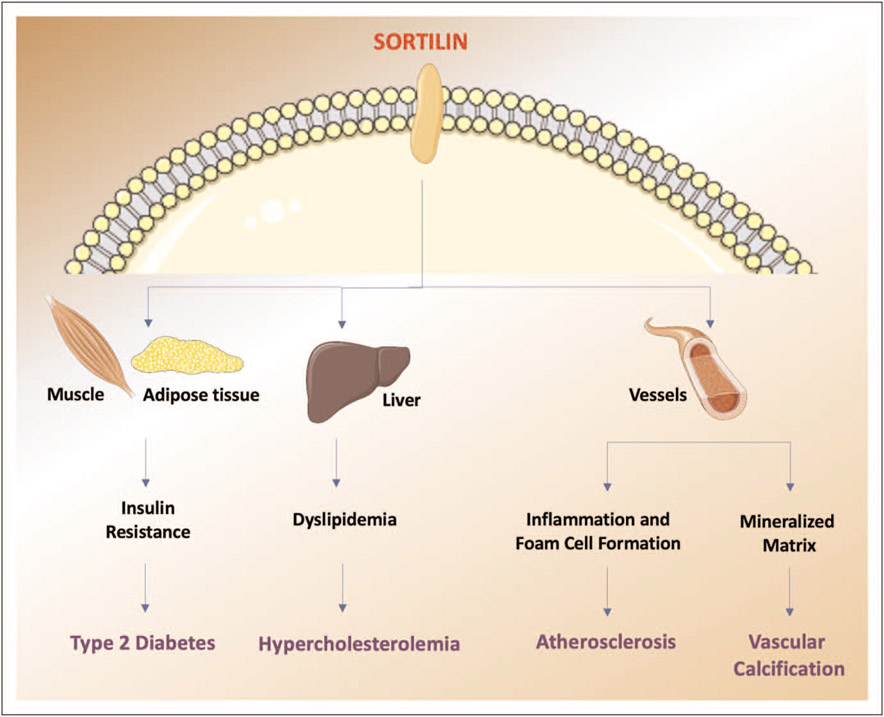
Since Sortilin contributes to the risk of hypertension and in general to an increased cardiovascular risk (Fig. 3), it could also be exploited as a potential therapeutic target. It is noteworthy to emphasize that a hypothetical therapy targeting Sortilin may have negative effects on the nervous system, as Sortilin is essential for a proper neuronal activity, inasmuch as it controls protein traffic and the release of neurotrophin, and also affects the signaling pathways governing cell survival and death via p75NTR [69■]. On the other hand, some studies have identified Sortilin as a risk factor for neurogenerative diseases, such as Alzheimer’s [70]. Of course, additional studies will be needed to better understand the complex biology of Sortilin in the sorting and signaling of proteins.
FIGURE 3.
Impact of Sortilin on cardiovascular risk. Sortilin influences cardiovascular risk acting on several organs and through different pathways.
There are still many limitations regarding the implications of this protein in hypertension and further multicenter and prospective follow-up studies will be needed; notwithstanding, in the future Sortilin could play a decisive role in fighting hypertension and placing new clinical perspectives for the development of new successful therapeutic approaches.
KEY POINTS.
Sortilin is a transmembrane glycoprotein mainly involved in the intracellular transport between the Golgi apparatus, the lysosomes, and the plasma membrane.
Circulating levels of Sortilin have been associated with a higher risk of major adverse cardiac and cerebrovascular events.
Sortilin has recently been shown to play an essential role in the control of vascular function, promoting endothelial dysfunction and hypertension.
In endothelial cells, Sortilin alters the sphingolipid/ceramide homeostasis, initiating a signaling cascade that from sphingosine-1-phosphate leads to increased oxidative stress.
Acknowledgements
We thank Dr. Wang and Dr. Gambardella for helpful discussion.
Financial support and sponsorship
The Santulli’s Lab is supported in part by the National Institutes of Health (NIH): National Heart, Lung, and Blood Institute (NHLBI: R01-HL164772, R01-HL159062, R01-HL146691, T32-HL144456), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK: R01-DK123259, R01-DK033823) to G.S., National Center for Advancing Translational Sciences (NCATS: UL1TR002556-06) to G.S., by the Diabetes Action Research and Education Foundation (to G.S.), and by the Monique Weill-Caulier and Irma T. Hirschl Trusts (to G.S.). S.S.J. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-21POST836407); F.V. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-22POST995561). U.K. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-23POST1026190).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Razo C, Welgan CA, Johnson CO, et al. Effects of elevated systolic blood pressure on ischemic heart disease: a Burden of Proof study. Nat Med 2022; 28:2056–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin Q, Ye T, Ye P, et al. Hypertension in stroke survivors and associations with national premature stroke mortality: data for 2.5 million participants from multinational screening campaigns. Lancet Glob Health 2022; 10: e1141–e1149. [DOI] [PubMed] [Google Scholar]
- 3.Breeyear JH, Shuey MM, Edwards TL, et al. Blood pressure polygenic scores are associated with apparent treatment-resistant hypertension. Circ Genom Precis Med 2022; 15:e003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurniansyah N, Goodman MO, Kelly TN, et al. A multiethnic polygenic risk score is associated with hypertension prevalence and progression throughout adulthood. Nat Commun 2022; 13:3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujii R, Hishida A, Nakatochi M, et al. Associations of genome-wide polygenic risk score and risk factors with hypertension in a Japanese population. Circ Genom Precis Med 2022; 15:e003612. [DOI] [PubMed] [Google Scholar]
- 6.Maj C, Salvi E, Citterio L, et al. Dissecting the polygenic basis of primary hypertension: identification of key pathway-specific components. Front Cardiovasc Med 2022; 9:814502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostchega Y, Hughes JP, Kit B, et al. Differences in hypertension and stage II hypertension by demographic and risk factors, obtained by two different protocols in US adults: National Health and Nutrition Examination Survey. Am J Hypertens 2022; 35:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forzano I, Mone P, Varzideh F, et al. The selective aldosterone synthase inhibitor Baxdrostat significantly lowers blood pressure in patients with resistant hypertension. Front Endocr 2022; 13:1097968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szyszka M, Skrzypczyk P, Stelmaszczyk-Emmel A, et al. Serum periostin as a potential biomarker in pediatric patients with primary hypertension. J Clin Med 2021; 10:2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattina A, Geraci G, Zammuto M, et al. Resistive index of ophthalmic artery as an imaging biomarker of hypertension-related vascular and kidney damage. Biomark Med 2021; 15:1155–1166. [DOI] [PubMed] [Google Scholar]
- 11.Palmu J, Tikkanen E, Havulinna AS, et al. Comprehensive biomarker profiling of hypertension in 36985 Finnish individuals. J Hypertens 2022; 40:579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Q, Wang P, Cai Y, et al. Circulating MicroRNA-505 may serve as a prognostic biomarker for hypertension-associated endothelial dysfunction and inflammation. Front Cardiovasc Med 2022; 9:834121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi J, Ren Y, Liu S, et al. Circulating miR-3656 induces human umbilical vein endothelial cell injury by targeting eNOS and ADAMTS13: a novel biomarker for hypertension. J Hypertens 2022; 40:310–317. [DOI] [PubMed] [Google Scholar]
- 14.Pandey A, Patel KV, Vongpatanasin W, et al. Incorporation of biomarkers into risk assessment for allocation of antihypertensive medication according to the 2017 ACC/AHA high blood pressure guideline: a pooled cohort analysis. Circulation 2019; 140:2076–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sesso HD, Buring JE, Rifai N, et al. C-reactive protein and the risk of developing hypertension. JAMA 2003; 290:2945–2951. [DOI] [PubMed] [Google Scholar]
- 16.Vasan RS, Evans JC, Larson MG, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med 2004; 351:33–41. [DOI] [PubMed] [Google Scholar]
- 17.Mandel EI, Forman JP, Curhan GC, et al. Plasma bicarbonate and odds of incident hypertension. Am J Hypertens 2013; 26:1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varzideh F, Jankauskas SS, Kansakar U, et al. Sortilin drives hypertension by modulating sphingolipid/ceramide homeostasis and by triggering oxidative stress. J Clin Invest 2022; 132:e156624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richner M, Pallesen LT, Ulrichsen M, et al. Sortilin gates neurotensin and BDNF signaling to control peripheral neuropathic pain. Sci Adv 2019; 5: eaav9946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.■■. Goettsch C, Hutcheson JD, Aikawa M, et al. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J Clin Invest 2016; 126:1323–1336. This investigation demonstrated that Sortilin drives vascular calcification through a mechanism that involves its recruitment into extracellular vesicles.
- 21.Rabinowich L, Fishman S, Hubel E, et al. Sortilin deficiency improves the metabolic phenotype and reduces hepatic steatosis of mice subjected to diet-induced obesity. J Hepatol 2015; 62:175–181. [DOI] [PubMed] [Google Scholar]
- 22.Lefrancois S, Zeng J, Hassan JA, et al. The lysosomal trafficking of sphingolipid activator proteins (SAPs) is mediated by sortilin. EMBO J 2003; 22:6430–6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.■■. Mortensen MB, Kjolby M, Gunnersen S, et al. Targeting sortilin in immune cells reduces proinflammatory cytokines and atherosclerosis. J Clin Invest 2014; 124:5317–5322. These data demonstrate that Sortilin finely regulates cytokine secretion and that targeting Sortilin in immune cells significantly attenuates inflammation, eventually reducing atherosclerosis.
- 24.Leloup N, Lossl P, Meijer DH, et al. Low pH-induced conformational change and dimerization of sortilin triggers endocytosed ligand release. Nat Commun 2017; 8:1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munck Petersen C, Nielsen MS, Jacobsen C, et al. Propeptide cleavage conditions sortilin/neurotensin receptor-3 for ligand binding. EMBO J 1999; 18:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazella J, Borsotto M, Heurteaux C. The involvement of Sortilin/NTSR3 in depression as the progenitor of spadin and its role in the membrane expression of TREK-1. Front Pharmacol 2018; 9:1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans SF, Irmady K, Ostrow K, et al. Neuronal brain-derived neurotrophic factor is synthesized in excess, with levels regulated by sortilin-mediated trafficking and lysosomal degradation. J Biol Chem 2011; 286:29556–29567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du H, Zhou X, Feng T, et al. Regulation of lysosomal trafficking of progranulin by sortilin and prosaposin. Brain Commun 2022; 4:fcab310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulrichsen M, Goncalves NP, Mohseni S, et al. Sortilin modulates schwann cell signaling and remak bundle regeneration following nerve injury. Front Cell Neurosci 2022; 16:856734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen CM, Nielsen MS, Nykjaer A, et al. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J Biol Chem 1997; 272:3599–3605. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z, Pace U, Ronen D, et al. Polypeptide gp95. A unique glycoprotein of olfactory cilia with transmembrane receptor properties. J Biol Chem 1986; 261:1299–1305. [PubMed] [Google Scholar]
- 32.Andersen JL, Schroder TJ, Christensen S, et al. Identification of the first small-molecule ligand of the neuronal receptor sortilin and structure determination of the receptor–ligand complex. Acta Crystallogr D Biol Crystallogr 2014; 70 (Pt 2):451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazella J. Sortilin/neurotensin receptor-3: a new tool to investigate neurotensin signaling and cellular trafficking? Cell Signal 2001; 13:1–6. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt V, Schulz N, Yan X, et al. SORLA facilitates insulin receptor signaling in adipocytes and exacerbates obesity. J Clin Invest 2016; 126:2706–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogawa K, Ueno T, Iwasaki T, et al. Soluble sortilin is released by activated platelets and its circulating levels are associated with cardiovascular risk factors. Atherosclerosis 2016; 249:110–115. [DOI] [PubMed] [Google Scholar]
- 36.Talbot H, Saada S, Naves T, et al. Regulatory roles of Sortilin and SorLA in immune-related processes. Front Pharmacol 2018; 9:1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demeule M, Charfi C, Currie J, et al. TH1902, a new docetaxel-peptide conjugate for the treatment of sortilin-positive triple-negative breast cancer. Cancer Sci 2021; 112:4317–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazella J Deciphering mechanisms of action of sortilin/neurotensin receptor-3 in the proliferation regulation of colorectal and other cancers. Int J Mol Sci 2022; 23:11888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goettsch C, Iwata H, Hutcheson JD, et al. Serum sortilin associates with aortic calcification and cardiovascular risk in men. Arterioscler Thromb Vasc Biol 2017; 37:1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giovannini S, Biscetti F, Brau F, et al. Sortilin/Omentin-1 ratio in peripheral artery disease: a cross-sectional study on 295 unselected elderly patients. Mech Ageing Dev 2022; 205:111677. [DOI] [PubMed] [Google Scholar]
- 41.Biscetti F, Nardella E, Rando MM, et al. Sortilin levels correlate with major cardiovascular events of diabetic patients with peripheral artery disease following revascularization: a prospective study. Cardiovasc Diabetol 2020; 19:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.■. Jankowski V, Saritas T, Kjolby M, et al. Carbamylated sortilin associates with cardiovascular calcification in patients with chronic kidney disease. Kidney Int 2022; 101:574–584. This clinical study identified carbamylation of circulating Sortilin as a risk factor for cardiovascular calcification in patients with CKD.
- 43.Oh TJ, Ahn CH, Kim B, et al. Circulating sortilin level as a potential biomarker for coronary atherosclerosis and diabetes mellitus. Cardiovasc Diabetol 2017; 16:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han W, Wei Z, Zhang H, et al. The association between sortilin and inflammation in patients with coronary heart disease. J Inflamm Res 2020; 13:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kjolby M, Andersen OM, Breiderhoff T, et al. Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export. Cell Metab 2010; 12:213–223. [DOI] [PubMed] [Google Scholar]
- 46.Moller PL, Rohde PD, Winther S, et al. Sortilin as a biomarker for cardiovascular disease revisited. Front Cardiovasc Med 2021; 8:652584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Werida RH, Omran A, El-Khodary NM. Sortilin and homocysteine as potential biomarkers for coronary artery diseases. Int J Gen Med 2021; 14:6167–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel KM, Strong A, Tohyama J, et al. Macrophage sortilin promotes LDL uptake, foam cell formation, and atherosclerosis. Circ Res 2015; 116:789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simsek Z, Alizade E, Guner A, et al. Correlation between serum sortilin levels and severity of extracranial carotid artery stenosis. Int J Clin Pract 2021; 75: e14733. [DOI] [PubMed] [Google Scholar]
- 50.Krahel JA, Baran A, Kaminski TW, et al. Proprotein convertase subtilisin/kexin type 9, angiopoietin-like protein 8, sortilin, and cholesteryl ester transfer protein-friends of foes for psoriatic patients at the risk of developing cardiometabolic syndrome? Int J Mol Sci 2020; 21:3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.■■. Di Pietro P, Carrizzo A, Sommella E, et al. Targeting the ASMase/S1P pathway protects from sortilin-evoked vascular damage in hypertension. J Clin Invest 2022; 132:e146343. This paper provides the first demonstration of the molecular mechanisms linking Sortilin and endothelial dysfunction in hypertension.
- 52.Chu X, Liu R, Li C, et al. The association of plasma sortilin with essential hypertension and subclinical carotid atherosclerosis: a cross-sectional study. Front Cardiovasc Med 2022; 9:966890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Emdin CA, Anderson SG, Callender T, et al. Usual blood pressure, peripheral arterial disease, and vascular risk: cohort study of 4.2 million adults. BMJ 2015; 351:h4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schulz E, Gori T, Munzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res 2011; 34:665–673. [DOI] [PubMed] [Google Scholar]
- 55.Strong A, Ding Q, Edmondson AC, et al. Hepatic sortilin regulates both apolipoprotein B secretion and LDL catabolism. J Clin Invest 2012; 122:2807–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J, Matye DJ, Li T. Insulin resistance induces posttranslational hepatic sortilin 1 degradation in mice. J Biol Chem 2015; 290:11526–11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.■. Musunuru K, Strong A, Frank-Kamenetsky M, et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature 2010;466:714–719. A series of studies in human cohorts and human hepatocytes reveals that a noncoding polymorphism at the 1p13 locus, rs12740374, creates a C/EBP (CCAAT/enhancer binding protein) transcription factor binding site and alters the hepatic expression of the SORT1 gene.
- 58.Liu DJ, Peloso GM, Yu H, et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nat Genet 2017; 49:1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 2010; 466:707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vujkovic M, Keaton JM, Lynch JA, et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multiancestry meta-analysis. Nat Genet 2020; 52:680–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.■. Conlon DM, Schneider CV, Ko Y, et al. Sortilin restricts secretion of apolipoprotein B-100 by hepatocytes under stressed but not basal conditions. J Clin Invest 2022; 132:e144334. This study revealed that under stress conditions, hepatic sortilin directs apoB toward lysosomal degradation rather than secretion.
- 62.Santulli G, Jankauskas SS, Gambardella J. Inclisiran: a new milestone on the PCSK9 road to tackle cardiovascular risk. Eur Heart J Cardiovasc Pharmacother 2021; 7:e11–e12. [DOI] [PubMed] [Google Scholar]
- 63.Clark JR, Gemin M, Youssef A, et al. Sortilin enhances secretion of apolipoprotein(a) through effects on apolipoprotein B secretion and promotes uptake of lipoprotein(a). J Lipid Res 2022; 63:100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sparks RP, Arango AS, Jenkins JL, et al. An allosteric binding site on sortilin regulates the trafficking of VLDL, PCSK9, and LDLR in hepatocytes. Biochemistry 2020; 59:4321–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maeda S, Nobukuni T, Shimo-Onoda K, et al. Sortilin is upregulated during osteoblastic differentiation of mesenchymal stem cells and promotes extracellular matrix mineralization. J Cell Physiol 2002; 193:73–79. [DOI] [PubMed] [Google Scholar]
- 66.Huang S, Yu X, Wang H, et al. Elevated serum sortilin is related to carotid plaque concomitant with calcification. Biomark Med 2020; 14:381–389. [DOI] [PubMed] [Google Scholar]
- 67.Goettsch C, Hutcheson JD, Hagita S, et al. A single injection of gain-of-function mutant PCSK9 adeno-associated virus vector induces cardiovascular calcification in mice with no genetic modification. Atherosclerosis 2016; 251:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol 2020; 16:223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.■. Nykjaer A, Lee R, Teng KK, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature 2004; 427:843–848. This seminal paper demonstrated that Sortilin is crucial in mediating the signaling pathway induced by the precursor of the nerve growth factor.
- 70.Xu SY, Jiang J, Pan A, et al. Sortilin: a new player in dementia and Alzheimertype neuropathology. Biochem Cell Biol 2018; 96:491–497. [DOI] [PubMed] [Google Scholar]