Abstract
Activity changes in the ipsi- and contralesional parietal cortex and abnormal interhemispheric connectivity between these regions are commonly observed after stroke, however, their significance for motor recovery remains poorly understood. We here assessed the contribution of ipsilesional and contralesional anterior intraparietal cortex (aIPS) for hand motor function in 18 recovered chronic stroke patients and 18 healthy control subjects using a multimodal assessment consisting of resting-state functional MRI, motor task functional MRI, online-repetitive transcranial magnetic stimulation (rTMS) interference, and 3D movement kinematics. Effects were compared against two control stimulation sites, i.e. contralesional M1 and a sham stimulation condition.
We found that patients with good motor outcome compared to patients with more substantial residual deficits featured increased resting-state connectivity between ipsilesional aIPS and contralesional aIPS as well as between ipsilesional aIPS and dorsal premotor cortex. Moreover, interhemispheric connectivity between ipsilesional M1 and contralesional M1 as well as ipsilesional aIPS and contralesional M1 correlated with better motor performance across tasks. TMS interference at individual aIPS and M1 coordinates led to differential effects depending on the motor task that was tested, i.e. index finger-tapping, rapid pointing movements, or a reach-grasp-lift task. Interfering with contralesional aIPS deteriorated the accuracy of grasping, especially in patients featuring higher connectivity between ipsi- and contralesional aIPS. In contrast, interference with the contralesional M1 led to impaired grasping speed in patients featuring higher connectivity between bilateral M1.
These findings suggest differential roles of contralesional M1 and aIPS for distinct aspects of recovered hand motor function, depending on the reorganization of interhemispheric connectivity.
Keywords: resting-state fMRI, rTMS, motor recovery, kinematics, intraparietal sulcus
Hensel et al. combine fMRI, rTMS and 3D hand movement analyses to test the contributions of contralesional motor cortex (M1) and anterior parietal sulcus (aIPS) to motor recovery post-stroke. The results reveal differential roles of M1 and aIPS in different aspects of recovered hand motor function, depending on interhemispheric connectivity.
See Ward (https://doi.org/10.1093/brain/awad001) for a scientific commentary on this article.
See Ward (https://doi.org/10.1093/brain/awad001) for a scientific commentary on this article.
Introduction
Stroke is a leading cause for long-term disability, causing persisting impairments in a majority of survivors.1,2 Neuroimaging and non-invasive brain stimulation studies have already been proven valuable to investigate the mechanisms of neural reorganization from a systems level perspective, with potential therapeutic applications arising from the possibility to modulate network reorganization by induction of neural plasticity to achieve more favourable outcomes.3,4 Consistent findings across studies are altered brain activation levels5–7 and connectivity patterns8–10 within and across the hemispheres. For the motor system, especially activity of in perilesional cortex in the primary motor cortex (M1), the dorsal premotor cortex (dPMC) and the anterior intraparietal sulcus (aIPS) were found to be enhanced when stroke patients move their stroke-affected hand.5
Repetitive transcranial magnetic stimulation (rTMS) allows us to investigate the causal contribution of one brain region for a given behaviour, when applied while participants perform a motor task.11–17 While most translational rTMS studies have focused on M1 when aiming to enhance the recovery in patients with hemiparesis, only few studies were published on the role of brain areas other than M1, such as the aIPS.18,19 Recent work has substantiated the idea of identifying new therapeutic stimulation targets outside the primary motor cortex, demonstrating the relevance of higher-order regions in the contralesional hemisphere, including the dPMC, the aIPS12,19 and the superior parietal lobule.15 Amongst these regions, the aIPS plays a key role in hand movements, given its involvement in visuomotor integration of hand motor tasks20,21 and goal-directed attention reallocation.22 The aIPS is anatomically situated in the rostral part of the intraparietal sulcus close to its junction to the postcentral sulcus. It forms a central hub within the frontoparietal network for online control of actions integrating representations of oneself and the environment, including fine-grained hand-object interactions.13,23,24 These functions are essential for upper limb rehabilitation after stroke, when especially goal-directed movements and interactions with objects are trained in order to restore patients’ abilities to engage in meaningful actions.25 Together, given the consistent bilateral recruitment of aIPS in paretic hand movements after stroke and its strategic position within frontoparietal regions involved in higher-level motor control and hand-object interaction in the healthy brain, the aIPS represents a target of interest for therapeutic neuromodulation especially regarding goal-directed actions such as grasping.
The relevance of the aIPS during motor tasks early after stroke was recently examined using an online-rTMS interference approach, demonstrating that disrupting intrinsic activity of contralesional aIPS by means of TMS pulse series administered during (‘online’) task execution induces a transient improvement of hand motor performance of the paretic hand.12,19 For example, we recently found that in the first days after stroke the influence of contralesional aIPS for movements of the stroke-affected hand was linked to the degree of interhemispheric connectivity between contralesional aIPS with ipsilesional aIPS as well as with ipsilesional M1.12 These findings point to a detrimental influence of the contralesional aIPS on hand motor performance of the stroke-affected hand already relatively early after the infarct. In contrast, motor performance of chronic stroke patients was impaired when applying online-rTMS in the superior parietal lobule,15 which in turn suggests a supportive role of regions in proximity to the aIPS months after stroke. With regards to the ipsilesional hemisphere, ipsilesional aIPS-M1 connectivity has been found to be increased after stroke,26,27 indicating that the ipsilesional aIPS is involved in the reorganization of the motor network during post-stroke recovery. However, data on whether or not aIPS holds a causal role for recovered hand motor function after stroke remain scarce.
Notably, the functional implication of altered activation within contralesional motor areas during hand movements may differ considerably across stroke patients, depending on multiple factors, in particular the severity of stroke impairment and the connectivity between motor-related regions.28–30 Stroke patients with more severe motor deficits typically feature enhanced motor activity already in the first few weeks after, but also return to normal activation levels in patients featuring successful motor recovery.6,31 Interhemispheric connectivity strengths between motor-related regions have been associated with better clinical motor scores, indicated by early increases of resting-state functional connectivity in patients with better motor outcome.10 On the other hand, dynamic causal modelling (DCM) revealed increased negative coupling from the contra- to the ipsilesional hemisphere in patients with poor recovery, and normalization of clinical performance accompanied by near to normal levels of connectivity.32 Yet, it remains to be elucidated if alterations of interhemispheric connectivity are the cause or the consequence of motor recovery.28,33 Given the supportive influence of contralesional brain regions,15 it appears likely that abnormalities of interhemispheric connectivity are relevant for a favourable motor outcome.
Two main hypotheses emerge from the findings reported above. First, patients with near-to-normal longitudinal outcome compared to patients with residual motor deficits show increased connectivity between motor areas. Second, the critical role of the contralesional aIPS in goal- and object-directed movements post-stroke depends on its indirect influence on the ipsilesional regions via interhemispheric connections.
To test these hypotheses, we applied a multimodal experimental setup comparing functional MRI (fMRI) connectivity with rTMS effects on hand movements in (i) contralesional aIPS; (ii) ipsilesional aIPS; (iii) contralesional M1; and (iv) a sham stimulation condition administered to the parieto-occipital vertex. Task fMRI was conducted to localize rTMS targets, resting-state fMRI allowed an assessment of functional connectivity between bilateral aIPS, M1 and dPMC, and online-rTMS during three different 3D-analysed hand movement tasks of increasing demands on hand object interaction (finger-tapping, pointing and grasping) characterized the effects caused by transient interference with the aforementioned target regions. To test our first hypothesis, functional connectivity between M1, aIPS and dPMC was compared between patients in order to detect different patterns of network alterations in patients with near-to-normal motor outcome and patients with more substantial residual deficits.10 These differences found in the functional connectivity domain were then used to test our second hypothesis, i.e. patients who showed better hand motor function were expected to depend more on the supportive influence of contralesional aIPS and M1, mediated via interhemispheric connectivity. As a result, transiently disrupting contralesional aIPS or M1 function by rTMS might result in the re-emergence of motor deficits. In particular, in patients with higher interhemispheric connectivity between these regions (M1-M1 and aIPS-aIPS), we expected an rTMS-induced deterioration of motor performance, given the putative recruitment of contralesional resources via transcallosal connections in patients with good motor outcome to maintain motor performance at the best possible level. We further expected that the roles of aIPS and M1, probed using online-rTMS depended on the investigated tasks given that already in healthy participants different tasks evoke different connectivity patterns between cortical areas.
Materials and methods
Participants
Nineteen chronic stroke patients were recruited and examined at the Department of Neurology, University Hospital Cologne. Inclusion criteria were: (i) 40 to 90 years of age; (ii) one single event of an ischaemic stroke, defined as being more than 6 months post-onset,34 having resulted in; (iii) an unilateral hand motor deficit. Exclusion criteria were (i) contraindications to TMS; (ii) contraindications to MRI; (iii) cerebral haemorrhage; (iv) bihemispheric infarcts; and (v) the presence of severe aphasia, apraxia, or neglect. In our final sample, minor non-motor symptoms during enrolment were found in five patients, four reporting mild hypesthesia and one presenting with mild aphasia. No signs of neglect could be detected in any of the patients.
One patient was unable to perform the motor tasks of the online-rTMS experiment as the hand motor deficit was stronger than expected. Consequently, this patient needed to be excluded. As a result, 18 right-handed patients [13 males, mean age 66.2 ± standard deviation (SD) 13.0, 30.4 ± 20.7 months post-stroke] were included in the final analysis (Table 1). As indicated by the National Institutes of Health Stroke Scale (NIHSS) hand items obtained in the acute as well as in the chronic phase post-stroke, patients’ upper limb function had recovered from a median motor impairment of 2.5 (range 1–4) during the acute phase to 1 (range 0–2) in the chronic phase (normal distribution was not assumed according to Shapiro–Wilks Test P < 0.05). Note that a difference of 1 represents a clinically meaningful improvement of arm motor abilities (e.g. arm drift against gravity versus no drift). As a healthy control group, 18 age- and gender-matched healthy participants (12 males; mean age 66.5 ± 7.2, P = 0.94) without neurological or psychiatric disease were recruited from our hospital’s database.
Table 1.
Sample characteristics
| Stroke patients | Healthy controls | df | P (t-test) | P (U-test) | P (χ2-test) | |
|---|---|---|---|---|---|---|
| Age, years | 66.2 ± 13.0 | 66.5 ± 7.2 | 26.7 | 0.937 | ||
| Gender, male/female | 13/5 | 12/6 | 0.717 | |||
| Lesion side, left/right | 8/10 | |||||
| EHI | 0.89 ± 0.15 | 0.78 ± 0.24 | 27.9 | 0.119 | ||
| Months post-stroke | 30.4 ± 20.7 | |||||
| Relative grip strength | 0.88 ± 0.20 | 1.02 ± 0.11 | 25.7 | 0.020 | ||
| ARAT affected handa | 55.5 (45.3–57.0) | 57.0 (57.0–57.0) | 17.0 | 0.007 | ||
| ARAT unaffected handa | 57.0 (57.0–57.0) | 57.0 (57.0–57.0) | n.a. | |||
| NIHSSa | 1.5 (1.0–2.8) | 0.0 (0.0–0.0) | 17.0 | <0.001 | ||
| NIHSS (acute phase)a | 7.0 (6.0–9.8) | |||||
| NIHSS (hand recovery)a | 1.5 (1.0–2.8) |
ARAT = Action Research Arm Test; df = degrees of freedom; EHI = Edinburgh Handedness Inventory; n.a. = not applicable (due to identical values across all subjects and groups); NIHSS (hand recovery) = difference of peak NIHSS Hand item scores during the first 4 days post-stroke and examination in our study; NIHSS = National Institutes of Health Stroke Scale. P-values < 0.05 are highlighted in bold.
Non-parametric data are presented as medians (IQR).
The following clinical scores were obtained on the day of the TMS session: stroke severity was assessed by the NIHSS,35 the Action Research Arm Test (ARAT) allowed us to rapidly and at the same time reliably assess upper limb function relevant for everyday movements, including grasp, grip and finger-thumb opposition.36 Additionally, the maximum grip strength was determined by a rubber ball vigorimeter (KLS Martin Group).12,37–39 To quantify the amount or recovery from the acute stroke phase, we obtained the peak value of each patient’s NIHSS score from the medical records assessed during the first 4 days after stroke onset. It is important to note that the aforementioned clinical measures were not considered sensitive enough to distinguish the behavioural response to online-rTMS, which we aimed to achieve with kinematic recordings of hand movements. Clinical scores allow us to relate patients of the present study to other cohorts or time points. In particular, the NIHSS is frequently obtained in the clinical routine featuring a high inter-rater reliability.40 In the present study the NIHSS was therefore used to compare stroke severity in the chronic phase to the initial motor impairment.
All participants provided informed written consent before inclusion. The study had been approved by the local ethics committee at the University of Cologne (file no: 17-244) and was performed following the Declaration of Helsinki (1969, last revision 2013).
Experimental strategy and stimulation targets
Based on our previous online-rTMS interference studies,12,16,19 we chose a single-blinded, randomized, control-group and control-stimulation controlled crossover study design. We used online-rTMS to interfere with each participant’s task fMRI-defined activation peaks in (i) ipsilesional; and (ii) contralesional aIPS; as well as (iii) the electrophysiological hot spot defined by TMS in contralesional M1 to examine changes of hand movement kinematics in tasks of different motor demands. The behavioural rTMS effects were finally related to fMRI functional connectivity acquired before the rTMS session.
The experiment was specifically designed to test our hypotheses that ipsi- and/or contralesional aIPS play a role for the longitudinal outcome of reaching and grasping movements. As a third stimulation target, we included the contralesional M1 as the yet most commonly used target of online-rTMS studies in stroke12,17,19,41; and furthermore (iv) a sham stimulation condition (see below). For each participant, the coordinates of the aIPS targets were defined individually by activity maps, derived from task fMRI data acquired before applying rTMS.12 As previous studies have shown that the TMS motor hot spot and fMRI activation maximum usually differ in the range of a few millimetres,42 we considered the motor hot spot to more directly represent the best location to interfere with M1 activity using rTMS. Hence, the stimulation site in the contralesional M1 was defined by the motor hot spot, i.e. the coil position eliciting motor evoked potentials (MEP) of the highest amplitudes. The location used for sham stimulation was derived from skull landmarks, i.e. the parieto-occipital vertex (corresponding to Pz in the 10-20 EEG system), with the coil angled at 45°, touching the skull not with the centre but with the rim opposite the handle. This arrangement warranted a relatively larger coil–cortex distance so that the electromagnetic field was too weak to cause a substantial modulation of cortical neurons, especially with respect to motor related processes.43,44
Data acquisition for each participant lasted in sum ∼4 h, including screening for exclusion criteria and obtaining informed consent, preparation before each visit (such as hardware check, preprocessing and upload of stimulation targets in neuronavigation software), clinical assessment, instruction with test trials, electrophysiological examinations, fMRI acquisition, and application of online-rTMS. To prevent exhaustion of participants and to allow for sufficient time to define the stimulation targets based on the blood oxygen level-dependent (BOLD) activation maxima, the experiment was structured in two visits, a first fMRI session and a second online-rTMS session. On average, the time between both sessions was 4.2 days in patients (±1.8, range 3–6 days) and 4.2 days in healthy participants (±2.3, range 1–7 days).
Tasks and kinematic assessment
The contribution of the cortical regions of interest for motor performance was probed by three tasks of increasing demands on hand object interaction, i.e. (i) finger-tapping; (ii) rapid pointing between to targets; and (iii) a reach-grasp-lift task (Fig. 1C).12,17,19 All patients performed the task with their stroke-affected hand, i.e., contralateral to the lesioned hemisphere. Notably, tapping tasks are relatively easy to implement under standardized experimental conditions, which is why most neuroimaging experiments employed tapping tasks to examining upper limb function after stroke.5 However, tapping performance does not necessarily reflect motor impairments under real world conditions.45 Hence, we implemented the pointing and reach-grasp-lift tasks, which were expected to increasingly involve aIPS function and to probe hand motor performance resembling activities of daily living. All movements were conducted with the impaired hand by patients or using the matched hand in healthy individuals.
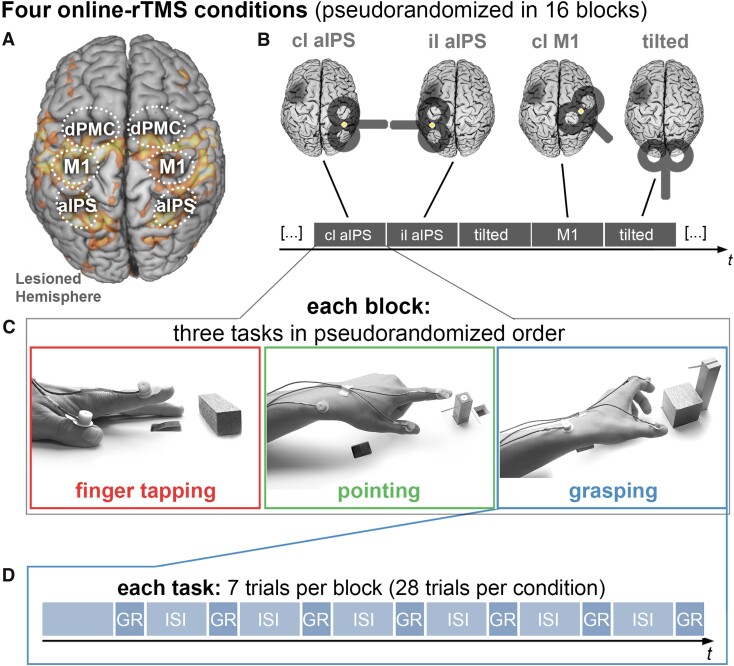
Figure 1.
Experimental setup. (A) Regions of interest for the connectivity analysis, drawn on an activation map of an example patient during paretic hand movements. (B) The online-rTMS session consisted of four rTMS conditions, each occurring in four blocks in a pseudo-randomized order. (C) In each block, participants performed three tasks: finger-tapping, pointing, and reach-grasp-lift an object. (D) Each task was performed in seven trials while rTMS was applied for 1.5 s after the onset of each trial. In total, each task was performed in 112 trials (28 trials per condition). Cl = contralesional; il = ipsilesional; GR = reach-grasp-lift condition; ISI = intertrial interval (5.5 s). Lesioned hemispheres are schematically marked by darkened lesion areas.
Behavioural performance was assessed by recording 3D movement kinematics using a high-resolution motion analyser system (Zebris). Each task was repeated 28 times per rTMS condition (contra- and ipsilesional aIPS, contralesional M1, sham stimulation), leading to 112 trials per task for the entire experiment. All trials were separated by breaks of 5.5 s to prevent fatigue and short-lasting carry-over effects of rTMS.46 Each movement was assessed with respect to four kinematic domains (i) efficiency; (ii) accuracy; (iii) smoothness; and (iv) speed.47 Details on each task and the kinematic assessment are provided in the Supplementary material.
Analysing four kinematic categories across three tasks yielded average performance values in 12 kinematic dimensions for each TMS condition. To compute an overall performance score across tasks and kinematic categories, we entered each participants’ 12 kinematic performance values (Fig. 2C) into a multiple factor analysis (MFA) using the FactoMineR package (version 1.39) implemented in R.48 In detail, assessing the mean efficiency, accuracy, smoothness and speed for three tasks (finger-tapping, pointing, reach-grasp-lift), resulted in 12 input variables, which we aimed to reduce to a score reflecting overall motor performance. The resulting first weighted principal component hence was defined to represent the motor composite score summarizing motor performance across tasks.49,50 Thereby, the MFA allowed us to quantify different dimensions of motor performance across tasks, which facilitated between-task comparisons. This analysis revealed that one dimension alone explained 47.1% of the total variance. Strikingly, this dimension was positively correlated with each kinematic feature—except for accuracy—across all tasks. In other words, participants with a high value in the first dimension performed more efficiently, smoother and faster, yet not more accurately. In contrast, the second and third dimensions did not reflect a clear pattern of motor performance, showing mixed correlations with kinematic features. We hence considered the first factor dimension as ‘kinematic motor composite score’ for all subsequent analyses.
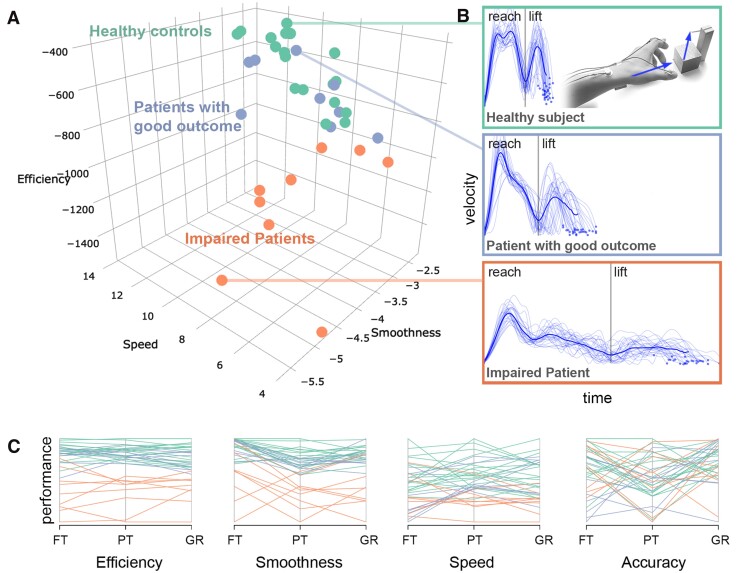
Figure 2.
Kinematic hand motor phenotypes. K-means clustering of hand kinematics yielded a two-cluster solution, (A) grouping healthy controls and patients with good motor outcome into the same cluster, in contrast to impaired patients. Participants are plotted based on reach-grasp-lift kinematics. (B) Examples of velocity profiles of one healthy participant (top), a patient with near-to-normal performance (middle), and one impaired patient (bottom). The dark blue lines indicate the mean velocity profile. (C) Parallel plot of all kinematic input variables entered into the clustering analysis, showing each participant’s performance for each scaled variable. FT = finger-tapping; PT = pointing; GR = grasping.
Patient subgroups: good outcome versus persistent deficits
We computed a k-means clustering analysis based on the kinematic data recorded during the rTMS sham condition to distinguish between patients with persistent deficits and patients with near-to-normal motor function (R stats and factoextra packages, www.r-project.org, version 3.4.3, for more details see Supplementary material). Therefore, the 12 kinematic features extracted from each of the 36 participants were z transformed and entered into a k-means clustering analysis with K = 2, aiming to differentiate patients with good motor outcome from patients with residual impairment based on their hand motor behaviour. Accordingly, a two cluster solution assigned nine patients into one cluster with all healthy participants representing a subgroup of patients with good motor outcome, whereas the nine remaining patients were grouped into a separate cluster, representing a subgroup with residual motor deficits.
MRI acquisition
Magnetic resonance images were acquired using a 3 T Magnetom Prisma scanner equipped with a 64-channel head coil (Siemens AG). A gradient echo planar imaging (EPI) multiband sequence was used with the following parameters: repetition time = 0.81 s, echo time = 0.030 s, field of view (FOV) = 212 mm, 72 axial slices, 2.0 mm3 isometric voxel size, flip angle = 52°. These parameters were equally used during the motor task (794 volumes) and resting-state (450 volumes). Stroke lesions were identified by T2-weighted images for all patients (repetition time = 3.2 s, echo time = 0.566 s, FOV = 241 mm, 208 axial slices, 0.94 mm3 isometric voxel size). Additionally, T1-images were acquired (MP RAGE, repetition time = 2.5 s, echo time = 2.22 ms, FOV = 241 mm, 208 axial slices, 0.94 mm3 isometric voxel size, flip angle = 7°) to screen for structural abnormalities and for EPI co-registration.
To localize individual activation maxima during finger-tapping with the stroke-affected hand, we employed a block design of 16 blocks in total. The MRI task consisted of visually cued repetitive finger-tapping movements with the left (eight blocks) and right hand (eight blocks).51 Written instructions displayed for 2.5 s indicated whether the left or right hand had to be moved in the upcoming block of trials. Each finger-tapping block included three trials (each lasting 3.5 s with a 3.2 s break to prevent fatigue,51,52 in which a visually presented arrow instructed participants to perform continuous index finger-tapping at maximal speed with the respective hand). Note that the fMRI task was equivalent to the rTMS finger-tapping task to facilitate comparability. All blocks were followed by a 16-s period of rest. Before the experiment started, all participants were trained until correctly performing the task. The total duration of the fMRI task lasted ten minutes and 43 s.
For resting-state fMRI, lasting 6 min and 5 s, participants were asked to open their eyes fixating on a cross presented on a black screen. After the session, all participants confirmed that they did not fall asleep during resting-state fMRI. We have shown in the past that this experimental setup allows a robust estimation of resting-state connectivity in the motor system in stroke patients.38,44,53
Imaging data were preprocessed and analysed using Statistical Parametric Mapping (SPM12; The Wellcome Centre for Human Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk) implemented in MATLAB version 2016b (The Mathworks Inc., MA, USA). Before preprocessing, the first five EPI scans were discarded as dummy images to prevent noise from magnetic field saturation. For all group analyses, images from patients with right-hemispheric lesions (n = 10) were flipped along the midsagittal plane so that the lesioned side corresponded to the left hemisphere in all participants.26,54 Correspondingly, data from nine healthy controls were flipped to account for systematic effects from hemispheric differences. Stroke lesions were delineated based on individual T2 maps using MRIcron (www.sph.sc.edu/comd/rorden/MRicron). To assess the lesion overlap across patients, lesion maps were co-registered and normalized into the MNI space using cost-function masking.
Analysis of task activation
Task fMRI data (794 volumes) were analysed on the single-subject level to localize individual activation peaks in both aIPS regions. Images were realigned, co-registered, and smoothed with a Gaussian filter of 4 mm full-width at half-maximum (FWHM). This filter size was used to identify the coordinates for the three TMS sites in individual participants.12 The general linear models (GLM) were computed using the six head motion parameters as covariates accounting for movement-related nuisance.
For the second level group analysis, task fMRI data were realigned, co-registered, spatially normalized to the standard template of the MNI, and smoothed by the default 8 mm FWHM Gaussian kernel to account for inter-subject anatomical variability. GLM contrast images were analysed at the second level using a full factorial ANOVA with the within-subject factor ‘Hand’ (levels affected, unaffected). The resulting T-maps (Fig. 3A) were thresholded at the voxel level (cluster forming threshold P < 0.001) and cluster-level corrected for multiple comparisons at P < 0.05 (family-wise error, FWE).
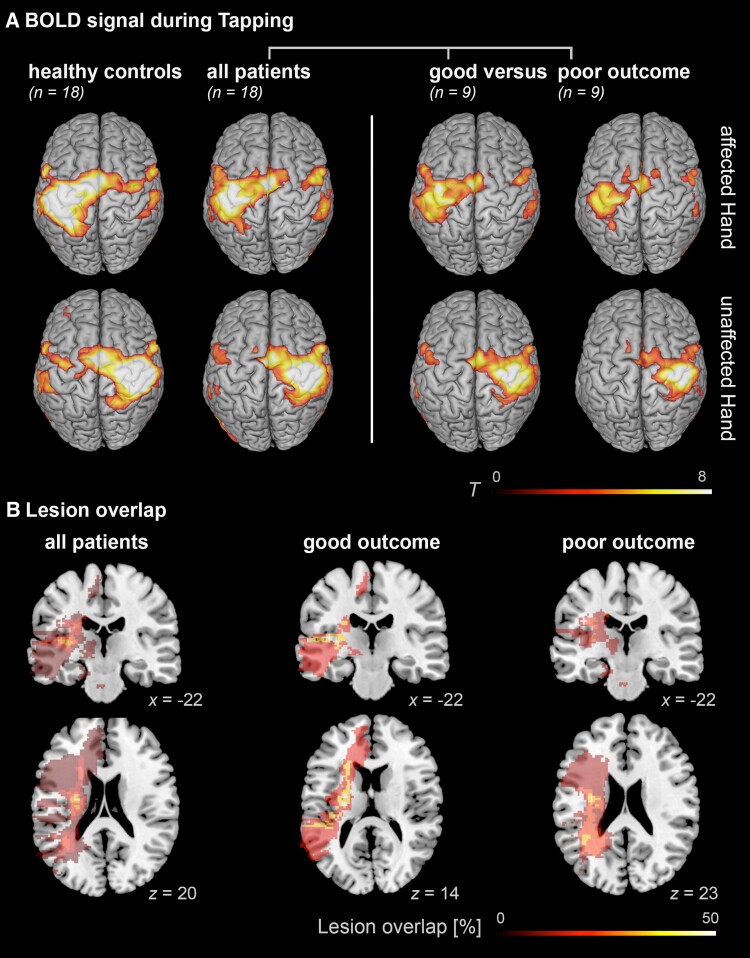
Figure 3.
Activation and lesion maps. (A) Group effects of BOLD activation indicating reduced activation in the ipsilesional M1 in impaired patients when tapping with the affected hand, whereas patients with good outcome show more activation of contralesional frontoparietal regions. Results were FWE cluster-level corrected at a threshold of P < 0.05 (cluster-forming threshold at the voxel level: P < 0.001). (B) Lesion overlap of stroke patients, based on T2 images. Lesion maps showed maximal overlap in the internal capsule in all patients as well as the two patient subgroups.
Resting-state functional connectivity
Preprocessing of the resting-state data (445 volumes) and extraction of single-subject connectivity was performed using MATLAB (version 2016b) and SPM12. Group analyses were computed using R (version 3.4.3). EPI volumes were spatially realigned to the mean image and co-registered with the structural T1-weighted image using SPM default parameters. Volumes were then corrected for head movement by affine registration using a two-pass procedure. The mean EPI image for each participant was spatially normalized to the MNI single-subject template55 using the ‘unified segmentation’ approach and applying the ensuing deformation to the individual EPI volumes.56 Next, images were smoothed by a 5 mm FWHM Gaussian kernel to improve signal-to-noise ratio and account for residual anatomical variations, consistent with our previous resting-state analyses.57,58 Within-scanner movements were corrected via regression of 24 movement parameters including the six motion parameters derived from the image realignment and their first derivative from the realignment as first and second order term,59,60 before bandpass filtering between 0.01 and 0.08 Hz.61 After preprocessing, resting-state functional connectivity was computed between regions of interest, i.e. M1, aIPS and dPMC, derived from an activation likelihood meta-analysis on hand movements after stroke.5 The six coordinates of this network comprising bilateral aIPS, M1, and dPMC were selected to define spherical seeds with a radius of 5 mm. Between these seeds, functional connectivity was estimated based on full Pearson correlations, which were subsequently z-transformed using the Fisher transformation of the r-values. Between these seeds, functional connectivity was estimated based on full Pearson correlations, which were subsequently z-transformed using the Fisher transformation of the r-values. Tract-tracing studies in macaques and tractography studies in humans have reported structural connections between these regions, consistently showing transcallosal connections between bilateral M1,62,63 dPMC64 and aIPS.64,65 Yet, it should be noted that while direct anatomical connections between the seeds are anatomically plausible, fMRI-based connectivity cannot exclude the possibility that the measured connectivity between two regions is mediated indirectly, via third-party regions.
Group comparisons of functional connectivity were performed by independent t-tests between patients and healthy controls and between both patient subgroups to identify motor performance-related connectivity patterns in stroke patients. Moreover, we computed Pearson correlations between connectivity of all patients and the first dimension derived from the multiple factor analyses, reflecting patients’ motor performance across all investigated tasks and features. All analyses were false discovery rate (FDR) corrected for multiple comparisons.65 The ensuing connections (Fig. 4) reflected performance-related alternations of the motor network after stroke, which we used as priors to test whether stroke specific connectivity alterations were linked to patients’ behavioural response to rTMS over contralesional M1 and aIPS.
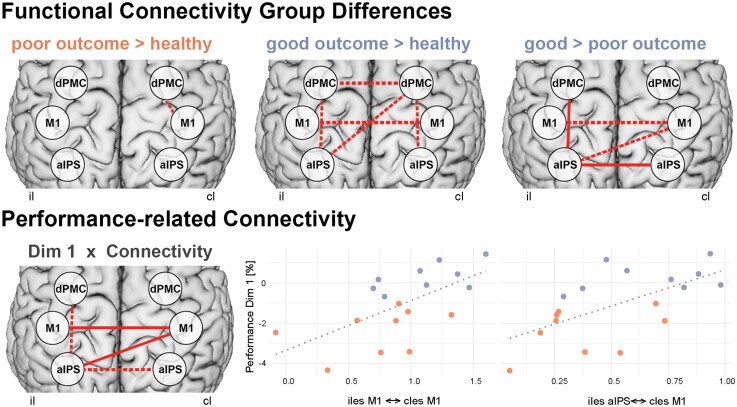
Figure 4.
Stroke-related connectivity alterations. Top: Higher frontoparietal and interhemispheric connectivity was found for patients with good motor outcome, compared to healthy controls and compared to patients with poor outcome. Bottom: Correlating connectivity with motor performance across tasks (dimension 1 of the multiple factor analysis) revealed similar connections related to motor performance after stroke. Solid lines indicate FDR-corrected results P < 0.05. Dotted lines indicate uncorrected results P < 0.05. Orange = impaired patients; blue = patients with good motor outcome.
Neuronavigated rTMS
TMS was applied using a Magstim® Super Rapid2 system (The Magstim Co. Ltd.) equipped with a Magstim® 70 mm Double Air Film Coil. Coil positions were navigated with a frameless computerized stereotaxic system (Brainsight V.2.0.7, Rogue Research, Inc). Thereby, individual T1 images were co-registered with participants’ anatomical landmarks, increasing the precision of coil navigation about the stimulation targets.66–68 Resting motor thresholds (rMTs) were individually obtained for M1 ipsilateral to the hand investigated (i.e. contralesional M1 in patients and the stroke unaffected hand). Importantly, the stimulation intensity below the rMT prevented the induction of motor-evoked potentials, which otherwise could have confounded task performance. During motor task performance, rTMS was applied at 90% of the rMT, at a frequency of 10 Hz, time-locked to task execution at all stimulation conditions.12,17,19
The software Presentation® (Version 9.9, Neurobehavioral Systems, USA, http://www.neurobs.com) was used for stimulus presentation and triggering of rTMS.17 Each time a movement was performed, 16 rTMS pulses were applied at 10 Hz beginning after a visual and auditory start cue indicating the participant to start the respective motor task. The TMS coil position changed every seven trials in a pseudo-randomized order so that each of the locations was assessed for an equal number of trials during each quarter of the experiment to control for sequence and fatigue effects (Fig. 1).
Notably, in contrast to offline rTMS protocols, the chosen online-rTMS approach is considered to invariably interfere with the neural processing due to the relatively strong electromagnetic pulses administered at a high frequency, disturbing neural processing, i.e. transiently inducing a ‘virtual lesion’ and thereby affecting task performance.15,17,69–71 Further details on the rTMS parameters and the definition of rTMS targets are provided in the Supplementary material.
Statistics of online-rTMS effects
To assess regional contributions to motor performance in each participant, mean values of each kinematic feature (efficiency, accuracy, smoothness, speed) during real rTMS (verum conditions: ipsilesional M1, ipsilesional aIPS, contralesional aIPS) were normalized to kinematic measures of rTMS applied with the coil tilted over the parieto-occipital vertex (sham condition) using the formula .12,16 To test our hypothesis of connectivity-related roles of ipsi- and contralesional aIPS as well as contralesional M1, the normalized rTMS effects were used to test for correlations with stroke-related connectivity involving each task’s respective stimulation site and kinematic features of each task (Fig. 5). Correlation analyses were FDR-corrected for multiple comparisons.65
Figure 5.
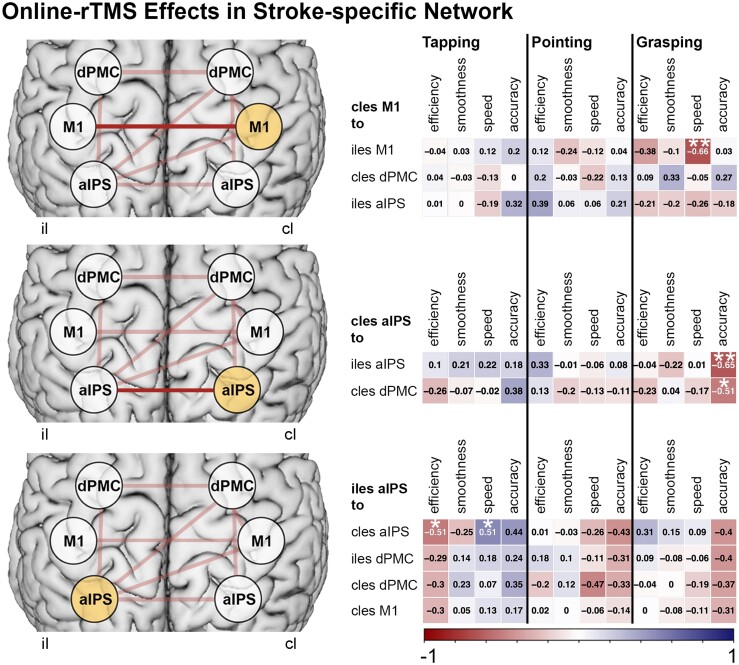
Online-rTMS effects. Stroke-specific connections (top row, connections in light red) with each stimulation target were tested for correlations with patients’ performance changes of kinematics during rTMS. This analysis revealed interhemispheric connections (top row, connections in dark red) to be related to the modulation of grasping kinematics during rTMS over contralesional M1 and aIPS. **FDR-corrected correlations P < 0.05. *Uncorrected correlations P < 0.05.
To test for region-specific rTMS effects at the group-level, normalized rTMS responses were entered into a four-way repeated measures ANOVA using the rstatix package (version 0.7.0) in R with the between-subject factor ‘Group’ (levels: ‘healthy controls’, ‘patients with good motor outcome’, ‘patients with residual impairment’) and the within-subject factors TMS ‘Region’ (three levels: ‘contralesional aIPS’, ‘ipsilesional aIPS’, ‘contralesional M1’), ‘Task’ (three levels: ‘finger-tapping’, ‘pointing’, ‘grasping’), and kinematic feature (four levels: ‘efficiency’, ‘accuracy’, ‘smoothness’, ‘speed’). We only considered interaction effects when including the factor ‘TMS region’ given our interest in region-specific stimulation effects.
Data availability
The data collected and analysed in the present study comprising demographics, clinical scores, kinematic readouts under rTMS, and functional connectivity are available for academic purposes (https://github.com/LukasHensel/chronic_stroke_fc_tms).
Results
Sample
Motor performance of patients (n = 18) featured significantly lower efficiency, smoothness, and speed across all motor tasks, compared to healthy controls (Table 2). Only for accuracy, no between-group differences were found for pointing, and grasping movements. This indicates that patients followed the experimental instruction to perform the task as accurate as possible with their stroke affected hand.
Table 2.
Group differences of hand kinematics
| Stroke patients | Healthy controls | P (t-test)a | df | Tb | |
|---|---|---|---|---|---|
| Finger-tapping | |||||
| ȃEfficiency | −252.22 ± 82.77 | −173.58 ± 27.82 | 0.002 | 20.8 | 3.82 |
| ȃAccuracy | −8.70 ± 3.49 | −6.51 ± 2.28 | 0.041 | 29.3 | 2.22 |
| ȃSmoothness | −2.14 ± 0.16 | −2.04 ± 0.06 | 0.024 | 21.3 | 2.55 |
| ȃSpeed | 325.05 ± 93.54 | 428.22 ± 80.37 | 0.002 | 33.2 | 3.55 |
| Reach-to-point | |||||
| ȃEfficiency | −539.42 ± 213.46 | −336.63 ± 63.01 | 0.002 | 19.9 | 3.87 |
| ȃAccuracy | −20.47 ± 2.75 | −20.08 ± 2.60 | 0.668 | 33.9 | 0.43 |
| ȃSmoothness | −2.58 ± 0.65 | −1.84 ± 0.37 | <0.001 | 26.9 | 4.18 |
| ȃSpeed | 9.43 ± 2.47 | 12.54 ± 2.53 | 0.002 | 34.0 | 3.73 |
| Reach-Grasp-Lift | |||||
| ȃEfficiency | −906.60 ± 257.56 | −600.53 ± 136.59 | <0.001 | 25.9 | 4.45 |
| ȃAccuracy | −23.47 ± 15.97 | −19.46 ± 7.60 | 0.376 | 24.3 | 0.96 |
| ȃSmoothness | −3.65 ± 0.92 | −2.87 ± 0.26 | 0.003 | 19.8 | 3.47 |
| ȃSpeed | 7.79 ± 2.24 | 9.77 ± 2.01 | 0.012 | 33.6 | 2.80 |
Efficiency = inverted movement time (ms); accuracy = inverted error, measured by distance to target (mm); smoothness = inverted number of velocity peaks; speed = peak velocity (mm/s); df = degrees of freedom. P-values < 0.05 are highlighted in bold.
All P-values are FDR-corrected for multiple comparisons.
T-value of Welch’s t-test.
Clinical scores suggest that patients had experienced substantial recovery, showing a significantly lower median NIHSS of 1.5 [interquartile range (IQR) 1.8, 1.0–2.8] at study enrolment, compared to the acute phase after stroke (median 7.0, IQR 3.8, 6.0–9.8, dependent Mann–Whitney Test: P < 0.001). Importantly, the ARAT scores obtained in the chronic phase still showed mild-to-moderate residual deficits of upper limb function (median 55.5, IQR 11.7, 45.3–57.0), indicating that recovery was good but not perfect.
Resting motor thresholds assessed in the unaffected hemisphere did not differ between groups [patients: 50.6% maximum stimulator output (MSO) ± 7.9%; healthy controls 52.9% MSO ± 12.7%, independent t-test, t(28.4) = 0.65, P = 0.524].
Highest lesion overlap was found at the level of the internal capsule, i.e. a region with high density of corticospinal motor fibres (Fig. 3B). Lesions of each patient are displayed in the Supplementary Fig. 3.
Rating the degree of small vessel disease using the Fazekas score ranging from 0 (absence of lesions) to 3 (largely confluent white matter lesions)72 showed that most patients showed mild to moderate white matter changes with a median score of 1 (IQR 1.85, 0.25–2), in line with findings of larger cohorts of stroke patients.73,74
Patient subgroups based on hand kinematics
The optimal cluster solution favoured n = 2 subgroups (Fig. 2), discriminating patients with motor performance similar to healthy controls (high performance) and patients with considerable residual deficits (low performance). Comparing all 12 kinematic features post hoc by independent t-tests, FDR-corrected for multiple comparisons, yielded higher efficiency (P < 0.003) and smoothness (P < 0.033) for all tasks in the high-performance compared to the low-performance patient subgroup. Moreover, patients in the high-performance group showed higher movement speed during pointing (P = 0.017) and grasping (P = 0.017). No significant differences were found for accuracy during all tasks (P > 0.079) and speed of finger-tapping (P = 0.769). Correspondingly, clinical measures indicated differences between both groups. Subgroups differed in relative grip strength [high-performance group: 0.98 ± 0.14, low-performance group: 0.78 ± 0.2; independent t-test t(13.5) = 2.32, P = 0.036, Cohen’s d = 1.1].
Further, the degree of recovery in both subgroups could be demonstrated by the NIHSS upper limb motor score which are acquired as part of the clinical routine (0: no paresis; 1: slow drift of arm; 2: affected arm can be held up with some effort but cannot be maintained at the same height as unaffected arm; 3: affected arm can be moved sideways, but not against gravity; 4: no movement at all). In the acute phase after stroke, NIHSS upper limb motor scores showed that all patients had originally experienced a clinically relevant paresis of the upper limb, which did not significantly differ between subgroups (Mann–Whitney Test: P = 0.316, high performance: median 3.0, IQR 2.0, 2.0–4.0; low performance: median 2.0, IQR 1.0, 2.0–3.0). To calculate the degree of recovery, we subtracted the NIHSS upper limb scores from the chronic phase with those obtained during the acute phase post-stroke (Supplementary Fig. 2). A non-paired, one-sided Mann–Whitney Test showed that patients with better kinematic performance had undergone more substantial recovery compared to those with lower kinematic performance (high performance: 2, IQR 2, 1–3; low performance: 1, IQR 1, 1–2; P = 0.033). Therefore, we conclude that the high-performance group featured a stronger amount of motor recovery compared to the low-performance group.
Comparing NIHSS upper limb motor scores in the chronic phase indicated a difference of motor outcome between both groups, in line with the kinematic data (Mann–Whitney Test: P = 0.045, high performance: median 0.0, IQR 1.0, 0.0–1.0; low performance: median 1.0, IQR 0.0, 1.0–1.0). Yet, since the NIHSS upper limb score does not take into account dexterity and thereby poorly discriminates mild-to-moderate hand deficits, it is not surprising that the ARAT score, including everyday hand movements such as pinch and grasp, demonstrated a clearer difference between patient subgroups, showing a higher (i.e. better) ARAT score in the high-performance group (median 57.0, IQR 2.0, 55.0–57.0) compared to the low-performance group (median 44.0, IQR 19.0, 37.0–56.0) (Mann–Whitney test: P = 0.032). White matter changes rated by Fazekas scores did not differ between subgroups (Mann–Whitney test: high performance: 1, IQR 2, 0–2; low performance: 1, IQR 1, 1–2; P = 0.85).
Stroke-related connectivity alterations
Comparing the entire group of patients with the healthy control group did not yield statistically significant differences in connectivity (PFDR > 0.340). However, when comparing the patient subgroups according to their residual deficits, we found that patients with good versus poor motor outcome featured higher resting-state functional connectivity between ipsi- and contralateral aIPS [t(15.4) = 4.15, P = 0.001, PFDR = 0.012, Cohen’s d = 1.96] and between ipsilesional aIPS and dPMC [t(15.6) = 3.27, P = 0.005, PFDR = 0.037, Cohen’s d = 1.54; Fig. 4, top right]. These findings indicate that the stroke impairment was related to connectivity alterations involving the aIPS. Correspondingly, connectivity between the ipsilesional aIPS and contralesional M1 correlated with motor performance across all patients—as reflected by the motor composite score derived from the MFA (Pearson r = 0.62, P = 0.0062, PFDR = 0.046). Moreover, higher interhemispheric connectivity between bilateral M1 (Pearson r = 0.62, P = 0.0059, PFDR = 0.046) was related to better hand motor performance after stroke (Fig. 4, bottom).
In summary, our findings indicate that aIPS connectivity did not only differentiate between patients with good motor outcome versus those with persistent motor deficits; but also that higher interhemispheric aIPS-aIPS as well as M1-M1 connectivity were linked to better motor performance. To test the causal role of these areas for patients’ motor performance, we next correlated their connectivity strengths with behavioural responses to online-rTMS interference.
Connectivity-related rTMS effects
When comparing normalized rTMS responses of each region using a four-way ANOVA with the between-subject factor GROUP (levels: ‘healthy controls’, ‘patients with good motor outcome’, ‘patients with residual impairment’) and the within-subject factors ‘TMS region’ (‘contralesional aIPS’, ‘ipsilesional aIPS’, ‘contralesional M1’), ‘Task’ (‘finger-tapping’, ‘pointing’, ‘grasping’), and ‘Kinematic feature’ (‘efficiency’, ‘accuracy’, ‘smoothness’, ‘speed’), none of the interactions including the factor ‘TMS region’ passed the threshold for statistical significance (all P > 0.05). This finding indicates that at the group level, TMS effects could not be explained by the respective factors.
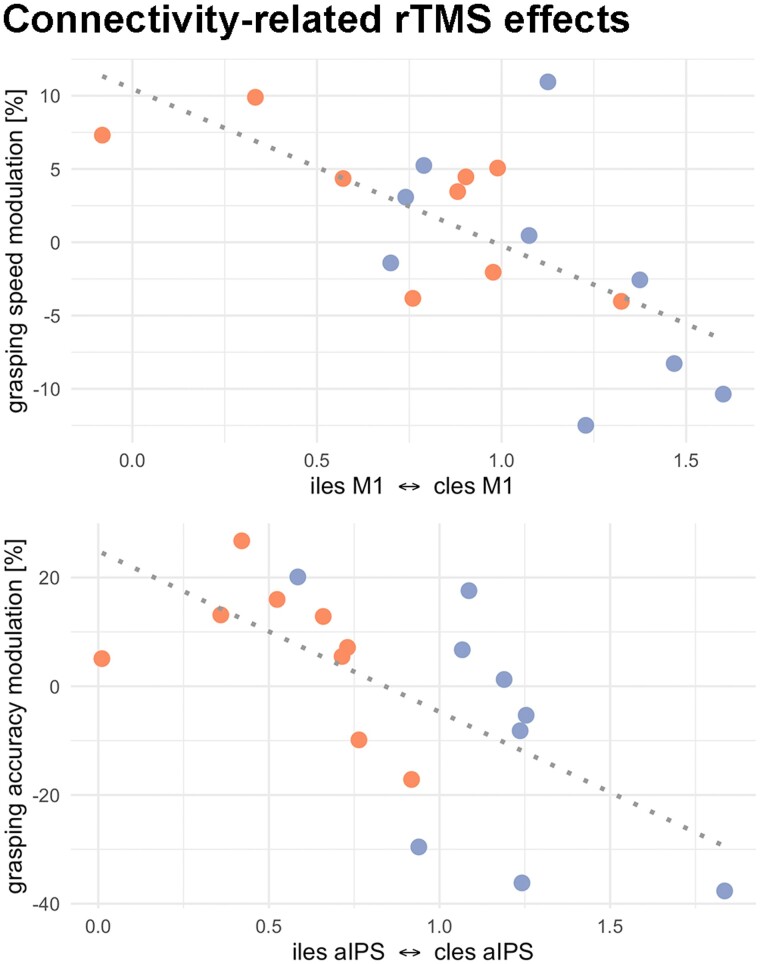
Given the group- and impairment-specific effects identified at the connectivity level reported above, we next aimed at linking TMS interference effects with the individual connectivity patterns. Hence, we correlated the region specific-rTMS responses with their respective patient-specific connectivity values. Regarding rTMS over aIPS, we observed a negative correlation between the rTMS effect on reach-grasp-lift accuracy and the interhemispheric connectivity between ipsilesional and contralesional aIPS (Pearson r = –0.65, P = 0.003, PFDR = 0.040). Hence, stronger interhemispheric connectivity between aIPS was linked to stronger rTMS effects upon contralesional aIPS interference for visuospatial grasp-to-lift performance. Here, patients featuring stronger interhemispheric aIPS connectivity showed more pronounced decreases in motor function during aIPS interference (Fig. 6). Concerning rTMS over contralesional M1, we found that that the alteration of grasping speed during rTMS was negatively correlated with the interhemispheric connectivity between both M1 (Pearson r = –0.66, P = 0.003, PFDR = 0.046). In other words, patients with stronger interhemispheric M1 connectivity were prone to a deterioration of grasping speed during rTMS applied to contralesional M1. None of the aforementioned correlations were observed in the healthy control group. Taken together, we demonstrate that rTMS modulates different movement kinematics, depending on clinical impairment and interhemispheric connectivity between bilateral M1 and aIPS, respectively.
Figure 6.
Connectivity-related rTMS effects. Top: Grasping speed was reduced during rTMS of contralesional M1 in participants with higher interhemispheric M1-connectivity. Bottom: Accuracy in the grasp-to-lift movement was reduced during rTMS of contralesional aIPS in participants with higher interhemispheric aIPS-connectivity. Orange = impaired patients; blue = patients with good motor outcome.
Discussion
Combining fMRI-guided online-rTMS, resting-state connectivity and kinematic analyses of hand movements, our data speak for a connectivity-related influence of contralesional M1 and contralesional aIPS for motor performance in recovered stroke patients. While replicating the involvement of interhemispheric M1-M1 connectivity in motor performance, our findings suggest that a supportive influence of the contralesional aIPS for more complex grasping movements after stroke relies on interhemispheric connectivity between bilateral aIPS, which differed significantly between patients with good versus poor motor outcome. Extending previous reports of altered interhemispheric connectivity in stroke,8,10,75 we here found higher levels of interhemispheric connectivity between bilateral aIPS and between ipsilesional aIPS and dPMC in patients with better motor performance. Interhemispheric connectivity between bilateral M1 correlated with higher motor performance after stroke as reflected by a motor composite score reflecting kinematic data from tasks of varying complexity (finger-tapping, pointing, grasping). Online-rTMS interference applied to contralesional aIPS resulted in impaired grasp-to-lift accuracy in patients with higher aIPS-aIPS connectivity, whereas rTMS applied to contralesional M1 deteriorated grasping speed in patients with higher M1-M1 connectivity. Hence, our data provide evidence that contralesional M1 and aIPS differentially support hand motor function in stroke patients with good motor outcome.
Performance-related connectivity after stroke
Longitudinal fMRI studies assessing functional connectivity after stroke have shown a complex, time- and impairment-dependent reorganization of functional connectivity after stroke.76,77 While most studies reported decreased connectivity between the hemispheres, especially in patients with chronic motor deficits several months and years after stroke,8,10,71,74–78 functional connectivity has been found to be restored towards levels of healthy controls10,78–80 or even higher81 in patients experiencing motor recovery. Previous findings, therefore, suggest an overall beneficial role of increased resting-state interhemispheric connectivity for the course of stroke recovery. The present findings do not only substantiate this hypothesis in chronic stroke patients, but also extend previous work by demonstrating the relevance of interhemispheric connectivity for motor performance in chronic stroke. Notably, enhanced resting-state connectivity was not restricted to M1, but also included aIPS connectivity. While previous connectivity studies examining hemiparetic stroke patients usually focused on abnormal connectivity in premotor regions and M1,76,77 a seed-to-whole brain analysis10 of functional connectivity showed that the ipsilesional M1 increasingly connects not only with contralesional M1 but also with bilateral parietal cortex including aIPS in the first months post-stroke. This result is compatible with our finding that connectivity between aIPS and M1 is linked to motor performance in chronic patients, indicating a relevant role for motor recovery.
The superior parietal lobule, adjacent to the intraparietal sulcus, has already been linked to motor recovery after stroke. For example, Wang and colleagues8 demonstrated that patients with higher connectivity between the contralesional superior parietal lobule and the ipsilesional dPMC were those with better recovery of the stroke-affected upper limb. Moreover, recent evidence from probabilistic tractography showed that the integrity of fibre tracts between the aIPS and the ventral premotor cortex correlates with motor function after stroke.82 Consistent with these findings, we here found higher levels of connectivity between both aIPS regions and between ipsilesional aIPS and dPMC in patients with good longitudinal outcome compared to lastingly impaired patients. Furthermore, interhemispheric connectivities between ipsilesional and contralesional M1 as well as between contralesional M1 and ipsilesional aIPS were correlated with patients’ motor performance. Thus, besides linking parietofrontal connectivity with motor performance after stroke, the present results indicate that the ipsilesional premotor cortex receives access to the contralesional motor-related system through an interhemispheric interaction between bilateral aIPS. This change in connectivity may help to explain previous evidence suggesting that the contralesional hemisphere reallocates resources to the ipsilesional hemisphere via cortico-cortical connections to facilitate the cortico-spinal output of the lesioned hemisphere.83 In line with this notion, previous data from task-fMRI demonstrated higher activation in frontoparietal regions including bilateral dPMC and aIPS during motor tasks already early after stroke84 This raises the question of whether bilateral recruitment of the parietal cortex reflects a potential compensatory mechanism or rather is a consequence of interhemispheric disinhibition.10 The present study allowed us to address this question by causally probing the roles of bilateral aIPS and relating their responses to aIPS-related functional connectivity.
Connectivity-related rTMS effects
We found a correlation between the behavioral TMS effects upon contralesional M1 or aIPS interference and their respective connectivity strengths with their ipsilesional counterparts. Accordingly, interfering with contralesional aIPS during grasping movements led to compromised accuracy during the grasp-to-lift task in patients with higher interhemispheric connectivity between bilateral aIPS. Our observation that the contralesional aIPS is implicated in grasping movements resonates well with the specialized role of the anterior aspects of intraparietal cortex in movement planning,85 visuomotor processing,86 and computation of complex hand-object interactions.13,23,24,87 Disrupting the aIPS contralateral to the moving hand in healthy participants has been shown to disturb hand shaping, grip force adaptation88,89 and online-control of goal directed movements.90 While—in healthy participants—online control of grasping has been shown to be primarily mediated by the aIPS contralateral to the moving hand (i.e. the ipsilesional aIPS),91 hand shaping during grasping could only be disturbed using bilateral rTMS over aIPS, providing evidence that dissociable features of unilateral grasping rely on a non-lateralized, effector-independent set of brain regions including aIPS.88,92 The non-lateralized nature of these areas coordinating synergies between hands, fingers and objects may be considered a promising basis for motor recovery after stroke, allowing contralesional regions to contribute to paretic hand movements. The present study suggests that particulary contralesional regions contribute to a favourable outcome of complex upper limb movements relevant for activities of daily living (pointing and grasping). Unlike in patients, rTMS responses of healthy participants did neither correlate with motor performance nor functional connectivity, suggesting that M1 and aIPS ipsilateral to the moving limb become increasingly relevant after stroke.
It should be noted that the aIPS represents one of the highest-order hubs connected to the motor system,93 which is involved in a wide range of functions beyond grasping, including tool use,94 motor attention,95 skill learning96 and goal interpretation in social context.97 Previous work by Carter and colleagues75 has provided first evidence that functional connectivity between parietal and frontal brain regions is related to motor performance after stroke. Strikingly, connectivity-related performance was not only found in visual attention tasks but also in motor tasks. In contrast, interhemispheric connectivity between primary sensorimotor regions was more domain-specific, correlating with motor performance, but not attentional abilities. The authors concluded that connections between higher-level frontoparietal regions might not only reallocate resources to visual stimuli, but also motor effectors.98,99 The present study confirms and extends these findings by probing the link between aIPS connectivity and motor performance in stroke patients. In particular, we demonstrated that spatial accuracy during the grasp-to-lift movement deteriorated increasingly in well performing patients with stronger bilateral aIPS connectivity when inducing a virtual lesion in the contralesional aIPS by rTMS. Thus, the present results reveal enhanced integrity between bilateral aIPS as a potential mechanism underlying a supportive contralesional aIPS function for the visuospatial abilities of patients during hand-object interactions.
Notably, rTMS over M1 did not affect spatial aspects of grasping performance, but lift-to-grasping speed, depending on the M1-M1 connectivity strengths (Fig. 5, left). One interpretation is that the contralesional M1 aids in normalizing the peak velocity of grasping in patients with higher bihemispheric M1 connectivity. Previous studies have examined hand motor performance applying rTMS over contralesional M1 during finger movements12,15,17 and pointing movements.19 Yet, while some of these studies indicate a detrimental influence of the contralesional M1 in the subacute phase after stroke,12,17 online-rTMS on M1 after three months post-stroke did not induce effects on tapping or pointing in most studies.17,19 Only data from Lotze and colleagues15 indicated a supportive role of M1 when observing more timing errors during complex finger movements during online-rTMS. By implementing tasks of varying demands, the present findings help to disentangle these contradicting effects of contralesional M1, suggesting no significant contributions during repetitive finger-tapping or pointing but during a more complex grasping task. Thus, our findings indicate a potentially supportive influence of M1 in chronic stroke dependent on motor outcome and task difficulty.
Neither connectivity of contralesional aIPS nor contralesional M1 were linked to rTMS effects in the other tasks (pointing and finger-tapping). These findings imply that interhemispheric connectivity with the contralesional M1 and aIPS is primarily relevant for movements with higher sensorimotor and visuospatial demands such as grasping and object interactions. Interestingly, interference with the ipsilesional aIPS tended to induce faster index finger velocity during the tapping task while slowing the movement cycle (deteriorating tapping efficiency) in patients with more robust bilateral aIPS connectivity (Fig. 5, right). This finding did not survive FDR correction for multiple comparisons. However, the relationship with rTMS effects on the rather simple finger-tapping task hints at a different contribution of interhemispheric connectivity to ipsilesional than contralesional aIPS.
Limitations
The study design requiring several repetitions of complex upper-limb movements excluded patients with severe hemiparesis or hemiplegia. Accordingly, our conclusion based on a sample with mild-to-moderate residual deficits cannot be transferred to patients with severe deficits. Furthermore, despite the fact that the sample size of the present study (n = 18) is among the largest reported for online-TMS studies in stroke patients, it is still relatively small to fully capture the large variability of stroke-induced changes. Further, the extensive behavioural assessment, including 3D kinematic recordings, fMRI, and online-rTMS, could only be performed in the chronic phase after stroke, whereas the assessment of behavioural deficits during the acute phase was limited to routine clinical scores. To further improve our understanding of the relationship between functional connectivity and the role of contralesional brain regions, longitudinal studies beginning in the acute phase after stroke are warranted.
Conclusion
The present study allowed to elucidate the behavioural roles of the contralesional M1 and aIPS for motor function after stroke in relation to elevated interhemispheric connectivity, thereby offering a mechanistic explanation of how these regions support higher motor abilities in chronic stroke patients with good motor outcome. These findings critically extend previous knowledge on the role of the aIPS in chronic stroke, indicating that higher-level resources from the contralesional aIPS are accessed via transcallosal connections, aiding complex hand movements in chronic stroke. Such information might help when aiming at identifying new therapeutic stimulation targets using non-invasive brain stimulation.
Supplementary Material
Contributor Information
Lukas Hensel, Faculty of Medicine and University Hospital Cologne, Department of Neurology, University of Cologne, Cologne, Germany.
Fabian Lange, Faculty of Medicine and University Hospital Cologne, Department of Neurology, University of Cologne, Cologne, Germany.
Caroline Tscherpel, Faculty of Medicine and University Hospital Cologne, Department of Neurology, University of Cologne, Cologne, Germany; Cognitive Neuroscience, Institute of Neuroscience and Medicine (INM-3), Research Centre Jülich, Jülich, Germany.
Shivakumar Viswanathan, Cognitive Neuroscience, Institute of Neuroscience and Medicine (INM-3), Research Centre Jülich, Jülich, Germany.
Jana Freytag, Faculty of Medicine and University Hospital Cologne, Department of Neurology, University of Cologne, Cologne, Germany.
Lukas J Volz, Faculty of Medicine and University Hospital Cologne, Department of Neurology, University of Cologne, Cologne, Germany.
Simon B Eickhoff, Institute of Systems Neuroscience, Medical Faculty, Heinrich Heine University Düsseldorf, Düsseldorf, Germany; Institute of Neuroscience and Medicine, Brain & Behaviour (INM-7), Research Centre Jülich, Jülich, Germany.
Gereon R Fink, Faculty of Medicine and University Hospital Cologne, Department of Neurology, University of Cologne, Cologne, Germany; Cognitive Neuroscience, Institute of Neuroscience and Medicine (INM-3), Research Centre Jülich, Jülich, Germany.
Christian Grefkes, Faculty of Medicine and University Hospital Cologne, Department of Neurology, University of Cologne, Cologne, Germany; Cognitive Neuroscience, Institute of Neuroscience and Medicine (INM-3), Research Centre Jülich, Jülich, Germany.
Funding
G.R.F. and C.G. are funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 431549029 – SFB 1451. G.R.F. gratefully acknowledges additional support from the Marga and Walter Boll Stiftung.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. GBD 2016 Neurology Collaborators . Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carod-Artal J, Egido JA, González JL, de Seijas E V. Quality of life among stroke survivors evaluated 1 year after stroke: experience of a stroke unit. Stroke. 2000;31:2995–3000. [DOI] [PubMed] [Google Scholar]
- 3. Grefkes C, Fink GR. Recovery from stroke: current concepts and future perspectives. Neurol Res Pract. 2020;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hensel L, Grefkes C, Tscherpel C, et al. . Intermittent theta burst stimulation applied during early rehabilitation after stroke: study protocol for a randomised controlled trial. BMJ Open. 2019;9:e034088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rehme AK, Eickhoff SB, Rottschy C, Fink GR, Grefkes C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. NeuroImage. 2012;59:2771–2782. [DOI] [PubMed] [Google Scholar]
- 6. Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiller C, Chollet F, Friston KJ, Wise RJS, Frackowiak RSJ. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol. 1992;31:463–472. [DOI] [PubMed] [Google Scholar]
- 8. Wang L, Yu C, Chen H, et al. . Dynamic functional reorganization of the motor execution network after stroke. Brain. 2010; 133:1224–1238. [DOI] [PubMed] [Google Scholar]
- 9. Fan Y-T, Wu C-Y, Liu H-L, Lin K-C, Wai Y-Y, Chen Y-L. Neuroplastic changes in resting-state functional connectivity after stroke rehabilitation. Front Hum Neurosci. 2015;9:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park C-H, Chang WH, Ohn SH, et al. . Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. 2011;42:1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG. The role of the human motor cortex in the control of complex and simple finger movement sequences. Brain. 1998;121:1695–1709. [DOI] [PubMed] [Google Scholar]
- 12. Hensel L, Tscherpel C, Freytag J, et al. . Connectivity-related roles of contralesional brain regions for motor performance early after stroke. Cerebral Cortex. 2021;31:993–1007. [DOI] [PubMed] [Google Scholar]
- 13. Koch G, Rothwell JC. TMS investigations into the task-dependent functional interplay between human posterior parietal and motor cortex. Behav Brain Res. 2009;202:147–152. [DOI] [PubMed] [Google Scholar]
- 14. Leone AP, Gates JR, Dhuna A. Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology. 1991;41:697–702. [DOI] [PubMed] [Google Scholar]
- 15. Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci. 2006;26:6096–6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tscherpel C, Hensel L, Lemberg K, et al. . Age affects the contribution of ipsilateral brain regions to movement kinematics. Hum Brain Mapp. 2020;41:640–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Volz LJ, Vollmer M, Michely J, Fink GR, Rothwell JC, Grefkes C. Time-dependent functional role of the contralesional motor cortex after stroke. Neuroimage Clin. 2017;16:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Plow EB, Cunningham DA, Varnerin N, Machado A. Rethinking stimulation of the brain in stroke rehabilitation: why higher motor areas might be better alternatives for patients with greater impairments. Neuroscientist. 2015;21:225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tscherpel C, Hensel L, Lemberg K, et al. . The differential roles of contralesional frontoparietal areas in cortical reorganization after stroke. Brain Stimul. 2020;13:614–624. [DOI] [PubMed] [Google Scholar]
- 20. Grefkes C, Ritzl A, Zilles K, Fink GR. Human medial intraparietal cortex subserves visuomotor coordinate transformation. NeuroImage. 2004;23:1494–1506. [DOI] [PubMed] [Google Scholar]
- 21. Grafton ST, de C Hamilton AF. Evidence for a distributed hierarchy of action representation in the brain. Hum Mov Sci. 2007; 26:590–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. [DOI] [PubMed] [Google Scholar]
- 23. Binkofski FC, Klann J, Caspers S. Chapter 4. on the Neuroanatomy and Functional Role of the Inferior Parietal Lobule and Intraparietal Sulcus. Elsevier Inc.; 2015:35–48. [Google Scholar]
- 24. Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. J Anat. 2005;207:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nilsen DM, Gillen G, Geller D, Hreha K, Osei E, Saleem GT. Effectiveness of interventions to improve occupational performance of people with motor impairments after stroke: an evidence-based review. Am J Occup Ther. 2015;69:6901180030p1––6901180030. p9. [DOI] [PubMed] [Google Scholar]
- 26. Schulz R, Buchholz A, Frey BM, et al. . Enhanced effective connectivity between primary motor cortex and intraparietal sulcus in well-recovered stroke patients. Stroke. 2016;47:482–489. [DOI] [PubMed] [Google Scholar]
- 27. Bönstrup M, Schulz R, Schoen G, et al. . Parietofrontal network upregulation after motor stroke. Neuroimage Clin. 2018;18:720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koch PJ, Hummel FC. Toward precision medicine: tailoring interventional strategies based on noninvasive brain stimulation for motor recovery after stroke. Curr Opin Neurol. 2017;30:388–397. [DOI] [PubMed] [Google Scholar]
- 29. Grefkes C, Fink GR. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol. 2014;13:206–216. [DOI] [PubMed] [Google Scholar]
- 30. Plow EB, Sankarasubramanian V, Cunningham DA, et al. . Models to tailor brain stimulation therapies in stroke. Neural Plasticity. 2016; 2016:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tombari D, Loubinoux I, Pariente J, et al. . A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. NeuroImage. 2004;23:827–839. [DOI] [PubMed] [Google Scholar]
- 32. Rehme AK, Eickhoff SB, Wang LE, Fink GR, Grefkes C. Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. NeuroImage. 2011;55:1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. [DOI] [PubMed] [Google Scholar]
- 34. Bernhardt J, Hayward KS, Kwakkel G, et al. . Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce. Int J Stroke. 2017;12:444–450. [DOI] [PubMed] [Google Scholar]
- 35. Brott T, Adams HP, Olinger CP, et al. . Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. [DOI] [PubMed] [Google Scholar]
- 36. Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4:483. [DOI] [PubMed] [Google Scholar]
- 37. Rehme AK, Fink GR, Cramon von DY, Grefkes C. The role of the contralesional motor cortex for motor recovery in the early days after stroke assessed with longitudinal FMRI. Cerebral Cortex. 2011;21:756–768. [DOI] [PubMed] [Google Scholar]
- 38. Volz LJ, Rehme AK, Michely J, et al. . Shaping early reorganization of neural networks promotes motor function after stroke. Cerebral Cortex. 2016;26:2882–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kwakkel G, Lannin NA, Borschmann K, et al. . Standardized measurement of sensorimotor recovery in stroke trials: Consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Neurorehabil Neural Repair. 2017;31:784–792. [DOI] [PubMed] [Google Scholar]
- 40. Dewey HM, Donnan GA, Freeman EJ, et al. . Interrater reliability of the National Institutes of Health Stroke Scale: rating by neurologists and nurses in a community-based stroke incidence study. Cerebrovasc Dis. 1999;9:323–327. [DOI] [PubMed] [Google Scholar]
- 41. Lotze M, Sauseng P, Staudt M. Functional relevance of ipsilateral motor activation in congenital hemiparesis as tested by fMRI-navigated TMS. Exp Neurol. 2009;217:440–443. [DOI] [PubMed] [Google Scholar]
- 42. Diekhoff S, Uludağ K, Sparing R, et al. . Functional localization in the human brain: Gradient-echo, spin-echo, and arterial spin-labeling fMRI compared with neuronavigated TMS. Hum Brain Mapp. 2011;32:341–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Herwig U, Cárdenas-Morales L, Connemann BJ, Kammer T, Schönfeldt-Lecuona C. Sham or real—Post hoc estimation of stimulation condition in a randomized transcranial magnetic stimulation trial. Neuroscience Letters. 2010;471:30–33. [DOI] [PubMed] [Google Scholar]
- 44. Nettekoven C, Volz LJ, Kutscha M, et al. . Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. J Neurosci. 2014;34:6849–6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wulf G, Shea CH. Principles derived from the study of simple skills do not generalize to complex skill learning. Psychon Bull Rev. 2002;9:185–211. [DOI] [PubMed] [Google Scholar]
- 46. Rotenberg A, Horvath JC, Pascual-Leone A. The Transcranial Magnetic Stimulation (TMS) Device and Foundational Techniques. In: Transcranial Magnetic Stimulation. Vol 89. Neuromethods. New York, NY: Humana Press, New York, NY; 2014:3–13. [Google Scholar]
- 47. Schwarz A, Kanzler CM, Lambercy O, Luft AR, Veerbeek JM. Systematic review on kinematic assessments of upper limb movements after stroke. Stroke. 2019;50:718–727. [DOI] [PubMed] [Google Scholar]
- 48. Lê S, Josse J, Husson F. FactoMineR: an R package for multivariate analysis. J Stat Softw. 2008;25:1–18. [Google Scholar]
- 49. Escofier B, Pages J. Multiple factor analysis (AFMULT package). Comput Stat Data Anal. 1994;18:121–140. [Google Scholar]
- 50. Abdi H, Williams LJ, Valentin D. Multiple factor analysis: principal component analysis for multitable and multiblock data sets. WIREs Comp Stat. 2013;5:149–179. [Google Scholar]
- 51. Wang LE, Fink GR, Diekhoff S, Rehme AK, Eickhoff SB, Grefkes C. Noradrenergic enhancement improves motor network connectivity in stroke patients. Ann Neurol. 2011;69:375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sano Y, Kandori A, Shima K, et al. Reliability of finger tapping test used in diagnosis of movement disorders. 2011 5th Int Conf Bioinform Biomed Eng. Published online December 28, 2010:1–4. doi: 10.1109/icbbe.2011.5780409 [DOI]
- 53. Bonkhoff AK, Espinoza FA, Gazula H, et al. . Acute ischaemic stroke alters the brain's preference for distinct dynamic connectivity states. Brain. 2020;143:1525–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rehme AK, Volz LJ, Feis D-L, Eickhoff SB, Fink GR, Grefkes C. Individual prediction of chronic motor outcome in the acute post-stroke stage: Behavioral parameters versus functional imaging. Hum Brain Mapp. 2015;36:4553–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. [DOI] [PubMed] [Google Scholar]
- 56. Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. [DOI] [PubMed] [Google Scholar]
- 57. Eickhoff SB, Laird AR, Fox PT, Bzdok D, Hensel L. Functional segregation of the human dorsomedial prefrontal cortex. Cerebral Cortex. 2016;26:304–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hensel L, Hoffstaedter F, Caspers J, et al. . Functional connectivity changes of key regions for motor initiation in Parkinson’s disease. Cereb Cortex. 2018;29:383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. [DOI] [PubMed] [Google Scholar]
- 60. Satterthwaite TD, Elliott MA, Gerraty RT, et al. . An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage. 2013;64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cordes D, Haughton VM, Arfanakis K, et al. . Frequencies contributing to functional connectivity in the cerebral cortex in ‘resting-state’ data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 62. Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu XH, Wiesendanger M. Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Exp Brain Res. 1994;102:227–243. [DOI] [PubMed] [Google Scholar]
- 63. Ruddy KL, Leemans A, Carson RG. Transcallosal connectivity of the human cortical motor network. Brain Struct Funct. 2016;222:1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Boussaoud D, Tanné-Gariépy J, Wannier T, Rouiller EM. Callosal connections of dorsal versus ventral premotor areas in the macaque monkey: a multiple retrograde tracing study. BMC Neurosci. 2005;6:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B. 1995;57:289.–. [Google Scholar]
- 66. Sparing R, Buelte D, Meister IG, Paus T, Fink GR. Transcranial magnetic stimulation and the challenge of coil placement: a comparison of conventional and stereotaxic neuronavigational strategies. Hum Brain Mapp. 2008;29:82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Julkunen P, Säisänen L, Danner N, et al. . Comparison of navigated and non-navigated transcranial magnetic stimulation for motor cortex mapping, motor threshold and motor evoked potentials. NeuroImage. 2009;44:790–795. [DOI] [PubMed] [Google Scholar]
- 68. Bashir S, Edwards D, Pascual-Leone A. Neuronavigation increases the physiologic and behavioral effects of low-frequency rTMS of primary motor cortex in healthy subjects. Brain Topogr. 2011;24:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Johansen-Berg H, Rushworth MFS, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci USA. 2002;99:14518–14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rossi S, Rossini PM. TMS in cognitive plasticity and the potential for rehabilitation. Trends Cogn Sci. 2004;8:273–279. [DOI] [PubMed] [Google Scholar]
- 71. Rossini PM, Burke D, Chen R, et al. . Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126:1071–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJNR Am J Neuroradiol. 1987;8:421–426. [DOI] [PubMed] [Google Scholar]
- 73. Melkas S, Sibolt G, Oksala NKJ, et al. . Extensive white matter changes predict stroke recurrence up to 5 years after a first-ever ischemic stroke. CED. 2012;34:191–198. [DOI] [PubMed] [Google Scholar]
- 74. Leys D, Englund E, Del Ser T, et al. . White matter changes in stroke patients. Relationship with stroke subtype and outcome. Eur Neurol. 1999;42:67–75. [DOI] [PubMed] [Google Scholar]
- 75. Carter AR, Astafiev SV, Lang CE, et al. . Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Desowska A, Turner DL. Dynamics of brain connectivity after stroke. Rev Neurosci. 2019;30:605–623. [DOI] [PubMed] [Google Scholar]
- 77. Rehme AK, Grefkes C. Cerebral network disorders after stroke: evidence from imaging-based connectivity analyses of active and resting brain states in humans. J Physiol. 2013;591:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Golestani A-M, Tymchuk S, Demchuk A, Goodyear BG. VISION-2 Study Group. Longitudinal evaluation of resting-state FMRI after acute stroke with hemiparesis. Neurorehabil Neural Repair. 2013;27:153–163. [DOI] [PubMed] [Google Scholar]
- 79. Liu H, Tian T, Qin W, Li K, Yu C. Contrasting evolutionary patterns of functional connectivity in sensorimotor and cognitive regions after stroke. Front Behav Neurosci. 2016;10:943–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xu H, Qin W, Chen H, Jiang L, Li K, Yu C. Contribution of the resting-state functional connectivity of the contralesional primary sensorimotor cortex to motor recovery after subcortical stroke. Wenderoth N, ed. PLoS One. 2014;9:e84729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zheng X, Sun L, Yin D, et al. . The plasticity of intrinsic functional connectivity patterns associated with rehabilitation intervention in chronic stroke patients. Neuroradiology. 2016;58:417–427. [DOI] [PubMed] [Google Scholar]
- 82. Schulz R, Koch P, Zimerman M, et al. . Parietofrontal motor pathways and their association with motor function after stroke. Brain. 2015;138:1949–1960. [DOI] [PubMed] [Google Scholar]
- 83. Gerloff C, Bushara K, Sailer A, et al. . Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129:791–808. [DOI] [PubMed] [Google Scholar]
- 84. Puh U, Vovk A, Sevšek F, Šuput D. Increased cognitive load during simple and complex motor tasks in acute stage after stroke. Int J Psychophysiol. 2007;63:173–180. [DOI] [PubMed] [Google Scholar]
- 85. Verhagen L, Dijkerman HC, Medendorp WP, Toni I. Hierarchical organization of parietofrontal circuits during goal-directed action. J Neurosci. 2013;33:6492–6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Grefkes C, Weiss PH, Zilles K, Fink GR. Crossmodal processing of object features in human anterior intraparietal cortex: An fMRI study implies equivalencies between humans and monkeys. Neuron. 2002;35:173–184. [DOI] [PubMed] [Google Scholar]
- 87. Tunik E, Frey SH, Grafton ST. Virtual lesions of the anterior intraparietal area disrupt goal-dependent on-line adjustments of grasp. Nat Neurosci. 2005;8:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Davare M, Andres M, Clerget E, Thonnard J-L, Olivier E. Temporal dissociation between hand shaping and grip force scaling in the anterior intraparietal area. J Neurosci. 2007;27:3974–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dafotakis M, Sparing R, Eickhoff SB, Fink GR, Nowak DA. On the role of the ventral premotor cortex and anterior intraparietal area for predictive and reactive scaling of grip force. Brain Res. 2008;1228:73–80. [DOI] [PubMed] [Google Scholar]
- 90. Rice NJ, Tunik E, Grafton ST. The anterior intraparietal sulcus mediates grasp execution, independent of requirement to update: new insights from transcranial magnetic stimulation. J Neurosci. 2006;26:8176–8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rice NJ, Tunik E, Cross ES, Grafton ST. On-line grasp control is mediated by the contralateral hemisphere. Brain Res. 2007;1175:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund H. A fronto-parietal circuit for object manipulation in man: evidence from an fMRI-study. Eur J Neurosci. 1999;11:3276–3286. [DOI] [PubMed] [Google Scholar]
- 93. Hamilton AF, Grafton ST. The motor hierarchy: from kinematics to goals and intentions. In: Haggard P, Rossetti Y, Kawato M, eds. Sensorimotor Foundations of Higher Cognition Attention and Performance XXII. Vol 1. Oxford; 2007. [Google Scholar]
- 94. Culham JC, Valyear KF. Human parietal cortex in action. Curr Opin Neurobiol. 2006;16:205–212. [DOI] [PubMed] [Google Scholar]
- 95. Rushworth MF, Krams M, Passingham RE. The attentional role of the left parietal cortex: the distinct lateralization and localization of motor attention in the human brain. J Cogn Neurosci. 2001;13:698–710. [DOI] [PubMed] [Google Scholar]
- 96. Tunik E, Rice NJ, Hamilton A, Grafton ST. Beyond grasping: representation of action in human anterior intraparietal sulcus. NeuroImage. 2007;36(Suppl 2):T77–T86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Frith CD, Frith U. Mechanisms of social cognition. Ann Rev Psychol 2012;63:287–313. [DOI] [PubMed] [Google Scholar]
- 98. Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rushworth MFS, Johansen-Berg H, Göbel SM, Devlin JT. The left parietal and premotor cortices: motor attention and selection. NeuroImage. 2003;20(Suppl 1):S89–S100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data collected and analysed in the present study comprising demographics, clinical scores, kinematic readouts under rTMS, and functional connectivity are available for academic purposes (https://github.com/LukasHensel/chronic_stroke_fc_tms).