Abstract
The neuroanatomical changes that underpin cognitive development are of major interest in neuroscience. Of the many aspects of neuroanatomy to consider, tertiary sulci are particularly attractive as they emerge last in gestation, show a protracted development after birth, and are either human- or hominoid-specific. Thus, they are ideal targets for exploring morphological-cognitive relationships with cognitive skills that also show protracted development such as working memory (WM). Yet, the relationship between sulcal morphology and WM is unknown—either in development or more generally. To fill this gap, we adopted a data-driven approach with cross-validation to examine the relationship between sulcal depth in lateral prefrontal cortex (LPFC) and verbal WM in 60 children and adolescents between ages 6 and 18. These analyses identified 9 left, and no right, LPFC sulci (of which 7 were tertiary) whose depth predicted verbal WM performance above and beyond the effect of age. Most of these sulci are located within and around contours of previously proposed functional parcellations of LPFC. This sulcal depth model outperformed models with age or cortical thickness. Together, these findings build empirical support for a classic theory that tertiary sulci serve as landmarks in association cortices that contribute to late-maturing human cognitive abilities.
Keywords: cortical folding, development, neuroanatomy, sulci, verbal working memory
Introduction
A fundamental point of exploration in cognitive neuroscience is the question of how brain anatomy gives rise to cognition. Exploring the developing human brain has provided some insights regarding brain-behavior relations. For instance, many magnetic resonance imaging (MRI) studies have examined how individual differences in cognitive development relate to cortical thickness, volume, and/or changes in white matter tissue properties (e.g. Shaw et al. 2006; Lu et al. 2007; Dickerson et al. 2008; Øtsby et al. 2011; Yeatman et al. 2012; Tamnes et al. 2013; Darki and Klingberg 2015; Wendelken et al. 2017; Bathelt et al. 2018). To a much lesser extent, previous studies have also examined the relationship between cognition and sulcal morphology in children (Gregory et al. 2016; Chung et al. 2017; Cachia et al. 2018; Tissier et al. 2018) despite the fact that cortical folding is a salient feature of human brain anatomy and its development (Chi et al. 1977; Welker 1990; White et al. 2010; Zilles et al. 2013). Importantly, little is known about the role in cognitive development—or cognition more broadly—of shallow, variable cortical indentations known as tertiary sulci, which often must be individually identified as a result of their variable location and morphology (Paus et al. 1996; Amiez et al. 2018; Petrides 2019).
Tertiary sulci are particularly interesting for 3 main reasons. First, they emerge late in gestation and continue to develop after birth (Sanides 1962, 1964; Welker 1990; Petrides 2019; Weiner 2019). Second, they are largely hominoid-specific structures and are particularly prominent in human association cortices such as lateral prefrontal cortex (LPFC), which supports higher-level cognition (Duncan and Owen 2000; Stuss and Knight 2002; Passingham and Wise 2012). Third, due to the protracted development of both tertiary sulci and higher order cognitive skills associated with LPFC, Sanides (1962, 1964) proposed a classic hypothesis that tertiary sulci likely serve as anatomical and functional landmarks that are behaviorally relevant in association cortices such as LPFC. In recent years, there has been mounting evidence in support of Sanides’ hypothesis in ventral temporal cortex (VTC; Weiner 2019), as well as in medial PFC (Lopez-Persem et al. 2019) and LPFC (Miller et al. 2021a; Voorhies et al. 2021). Directly relevant for the present study, the latter findings—supported by the notion that depth is a characteristic feature of tertiary sulci, which are much more shallow than primary and secondary sulci (Armstrong et al. 1995; Weiner 2019; Miller et al. 2021b)—showed that the depth of a subset of right-lateralized LPFC tertiary sulci correlated with performance on a visuospatial reasoning task.
Here, we build on those findings to test for a relationship between the depth of LPFC tertiary sulci and working memory (WM), a higher-level cognitive ability that depends on a broadly distributed brain network that includes LPFC (e.g. Paulesu et al. 1993; Petrides et al. 1993; Fiez et al. 1996; Nee and D'Esposito 2018). WM maintenance refers to the ability to keep mental representations active over the short-term: for example, rehearsing a phone number in verbal (or phonological) WM (Baddeley and Hitch 1974). In contrast, WM manipulation refers to the ability to reorganize or transform—i.e. “work with”—these active representations (Goldman-Rakic 1995). WM ability, particularly manipulation, improves over childhood and adolescence (Gathercole et al. 2004; Cowan 2016), and this improvement has been linked to LPFC development (Goldman-Rakic 1987; for review, see Bunge and Wright 2007). Here, we examine the role of LPFC sulcal morphology in verbal WM maintenance and manipulation.
Although neurophysiological and neuropsychological research has generally implicated LPFC in WM, this is a large and highly heterogeneous area. Functional brain imaging studies have reported functional dissociations along both the dorsal-ventral and rostral-caudal axes (Owen et al. 1996; D’Esposito et al. 1999; Koechlin et al. 2003; Fuster 2004; Petrides 2005; Badre 2008; Blumenfeld et al. 2013). With respect to verbal WM, specifically, neuroimaging studies have drawn a functional distinction between the portions of LPFC on either side of the inferior frontal sulcus (ifs): The inferior frontal gyrus (IFG; Brodmann areas 44, 45, and 47), also known as ventrolateral PFC (VLPFC), and the middle frontal gyrus (MFG; Brodmann areas 8, 9/46, and 46), or dorsolateral PFC (DLPFC).
In particular, left VLPFC (particularly Brodmann areas 44 and 45) has been consistently associated with verbal WM (Fiez et al. 1996; Smith and Jonides 1998; Wagner et al. 2001), with lesions affecting task performance when phonological rehearsal is required (Baldo and Dronkers 2006). In contrast, bilateral DLPFC (particularly area 9/46) has been implicated in control processes that operate on the contents of WM: that is, WM manipulation (Owen et al. 1996; D’Esposito et al. 1999; Sakai and Passingham 2003; Crone et al. 2006) and monitoring (Amiez and Petrides 2007). Currently, our understanding of the role of distinct LPFC subregions in verbal WM is limited by the fact that PFC lesions in humans tend to be quite large, and because insufficient attention is paid to sulcal anatomy in the WM neuroimaging literature (but see Amiez and Petrides 2007)—let alone to sulcal variation among individuals.
The present study examines the relationship between sulcal anatomy in LPFC and verbal WM in a sample of children and adolescents ranging in age from 6 to 18. This is a period of pronounced growth in WM, as well as large individual differences. Children show slower gains on tasks of WM manipulation than maintenance (Gathercole et al. 2004)—a pattern that our prior work has linked to protracted age-related changes in DLPFC (and superior parietal) activation during WM manipulation (Crone et al. 2006). This dataset provides an ideal sample for analyses of individual differences linking anatomy and behavior because the wide range of WM test scores observed across the sample is only partially attributable to age-related differences.
Based on our previous findings with regards to visuospatial reasoning (Voorhies et al. 2021), we hypothesized that the depth of a subset of LPFC sulci would be linked to verbal WM performance during development. We had 3 predictions. First, given Sanides’ hypothesis, we predicted that the depth of the shallow, late-developing tertiary sulci would be particularly predictive of individual differences in verbal WM. Second, given the ample evidence that verbal WM is left-hemisphere dominant, we predicted a leftward asymmetry in sulcal-cognitive relations. Third, given the previously documented dorsal-ventral dissociations within LPFC with regards to verbal WM, we predicted that sulci in VLPFC would be broadly implicated in verbal WM maintenance and manipulation, whereas those in DLPFC would be particularly linked to WM manipulation.
To directly address our predictions, we asked 3 main questions: (i) Is there a relationship between verbal WM maintenance and/or manipulation and mean depth of LPFC sulci? (ii) If so, do these relationships differ as a function of type of sulcus (shallow/tertiary or deep/non-tertiary), hemisphere, and/or task demands (WM maintenance and/or manipulation)? (iii) Can we construct a model to predict an individual’s verbal WM task score from sulcal depth? As described below, this study establishes a novel link between LPFC sulcal morphology and verbal WM skills in a pediatric cohort.
Materials and methods
Participants
Our present analyses leverage previously published data from the Neurodevelopment of Reasoning Ability (NORA) study (e.g. Wendelken et al. 2016, 2017). Sixty typically developing children and adolescents were randomly selected from the dataset for the purposes of manual sulcal labeling in individual brains. This sample size is appropriate based on previous work of this kind in humans (Weiner et al. 2014, 2018; Sprung-Much and Petrides 2018, 2020; Borne et al. 2020; Eichert et al. 2021; Miller et al. 2021a, 2021b; Voorhies et al. 2021), and given the detailed, labor-intensive nature of manually defining sulci on individual participants’ brains (a total of 2,157 sulcal labels in the present study).
Participants were all right-handed native English speakers, ranging in age from 6 to 18 (M = 12.12, SD = 3.39; Females: M = 11.82, SD = 3.23; Males: M = 12.36, SD = 3.50; see Supplementary Table 2 for additional demographic information). Only participants with at least one pimfs component in each hemisphere were included in our analyses (N = 57/60) so that all sulci were present in both hemispheres for each individual. Forty-eight of these participants were also included in our prior study (Voorhies et al. 2021).
All participants were screened for neurological impairments, psychiatric illness, history of learning disabilities, and developmental delay. All participants and their parents gave informed assent or consent to the study, which was approved by the Committee for Protection of Human Subjects at the University of California, Berkeley.
Behavioral measures
Verbal WM was measured via raw scores of the Digit Span test from the 4th edition of the Wechsler Intelligence Scale for Children (WISC-IV; Wechsler 1974). The Digit Span test—a standard measure of verbal WM—has been often used in studies investigating neural correlates of WM maintenance and manipulation (Li et al. 2012; Bathelt et al. 2018; Krogsrud et al. 2021). This task has two conditions: Digits Forward, which taxes WM maintenance, and Digits Backward, which taxes both WM maintenance and manipulation.
In Digits Forward, the experimenter reads aloud a sequence of single-digit numbers, and the participant is asked to immediately repeat the numbers in the same order; in Digits Backward, they are asked to immediately repeat the numbers in the reverse order. The length of the string of numbers increases after every 2 trials. The Forwards task has 8 levels, progressing from 2 to 9 digits (16 total trials). The Backwards task has 7 levels, from 2 to 8 digits (14 total trials). Participants are given a score of 1 for a correct answer or a 0 for an incorrect answer. Testing on a given task continues until a participant responds incorrectly to both trials at a given level, after which the experimenter recorded a score out of 16 for Digits Forward and a score out of 14 for Digits Backward.
MRI data
Brain imaging data were collected at the UC Berkeley Brain Imaging Center on a Siemens 3T Trio system. High-resolution T1-weighted MPRAGE anatomical scans (time repetition (TR) = 2,300 ms, time echo (TE) = 2.98 ms, 1 × 1 × 1-mm voxels) were acquired for cortical morphometric analyses.
Cortical surface reconstruction
All T1-weighted images were visually inspected for scanner artifacts. Using FreeSurfer’s automated segmentation tools (FreeSurfer 6.0.0: http://surfer.nmr.mgh.harvard.edu; Dale et al. 1999), each anatomical volume was segmented to separate gray from white matter, and the resulting boundary was used to reconstruct the cortical surface for each subject. Each reconstruction was visually inspected for segmentation errors and manually corrected when necessary.
Morphological analyses
Sulcal labeling
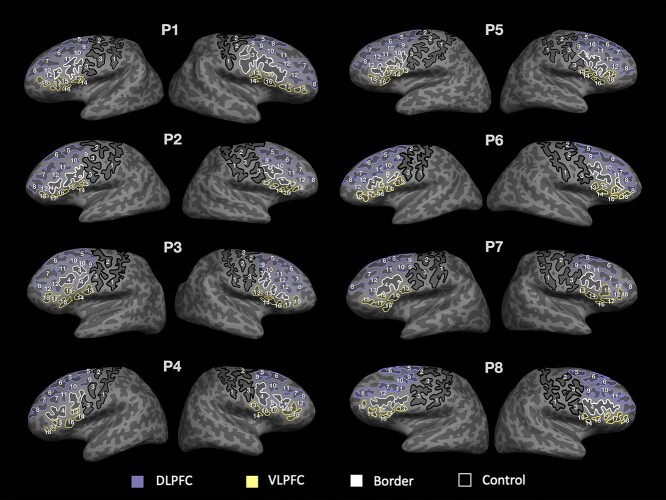
To account for intersubject variations in sulcal patterning, sulci were manually labeled on individual participants’ brains in native (i.e. unnormalized) space. Sulcal labels in LPFC were based on the most recent parcellation proposed by Petrides (2019; see also Sprung-Much and Petrides 2018, 2020). Regions defined as DLPFC and VLPFC are shown in relation to Brodmann areas on the Freesurfer fsaverage left-hemisphere inflated surface, as well as the cortical parcellation from Glasser et al. (2016; Supplementary Fig. 3c and d). In each hemisphere, 18 LPFC sulci in VLPFC and DLPFC were manually defined on both the pial and inflated surfaces (Fig. 1 and Supplementary Table 1), along with the ifs, referred to here as a border sulcus as it divides these regions. The imfs-v was also included in our definition of DLPFC, consistent with classic definitions of the middle frontal sulcus (Eberstaller 1890; see Miller et al. 2021b for a review of the classic literature); however, as we note elsewhere, this sulcus is now considered to be part of frontopolar cortex. As controls, we also labeled two precentral sulcal components and the central sulcus, which have not been linked to WM.
Fig. 1.
Sulcal definitions in LPFC in 6–18 year-olds. Inflated surfaces of the left and right hemispheres in 8 example participants (randomly chosen). Eighteen manually labeled sulci are outlined and labeled by number on each surface based on definitions derived from Petrides (2019). Eight sulci are included in DLPFC (purple) and 6 in VLPFC (yellow). The ifs (white) is considered the boundary between DLPFC and VLPFC. Sulci outlined in black (cs, sprs, and iprs) are not included in these regions and are considered as control sulci. The pimfs can be comprised of both a dorsal and a ventral component (RH: P1, P3, P5, and P7; LH: P1, P2, P3, P5, P6, and P7), include only one of these components (RH: P2 and P4; LH: P8), or be absent (RH: P6 and P8; LH: P4). See Supplementary Fig. 1 for all 2,157 sulcal definitions in all participants. See Supplementary Table 2 for demographic information for all participants. 1: central sulcus (cs); 2: superior precentral sulcus (sprs); 3: inferior precentral sulcus (iprs); 4: inferior frontal sulcus (ifs); 5: superior frontal sulcus—posterior (sfs-p); 6: superior frontal sulcus—anterior (sfs-a); 7: intermediate frontal sulcus—horizontal (imfs-h); 8: intermediate frontal sulcus—vertical (imfs-v); 9: posterior middle frontal sulcus—posterior (pmfs-p); 10: posterior middle frontal sulcus—intermediate (pmfs-i); 11: posterior middle frontal sulcus—anterior (pmfs-a); 12: paraintermediate frontal sulcus (pimfs); 13: diagonal sulcus (ds); 14: ascending ramus of the lateral fissure (aalf); 15: triangular sulcus (ts); 16: horizontal ramus of the lateral fissure (half); 17: pretriangular sulcus (prts); and 18: lateral frontomarginal sulcus (lfms).
The location and definition of each sulcus was identified separately in every individual by two trained independent raters (authors JKY and WIV). The raters compared independent ratings and confirmed the definitions. These sulcal definitions were then reviewed, further modified, and finalized by a neuroanatomist (KSW). The surface vertices for each sulcus were selected using tools in FreeSurfer and saved as surface labels for vertex-level analysis of morphological statistics. As it can sometimes be difficult to determine the precise start and end points of a sulcus on one surface (Borne et al. 2020), all definitions were also guided by the pial and smoothwm surfaces of each individual. Using multiple surfaces allowed us to form a consensus across surfaces and clearly determine each sulcal boundary as in our previous work (Miller et al. 2021a; Voorhies et al. 2021).
The dorsal and ventral components of the pimfs were not identifiable in all participants. However, we could identify at least one component in the right hemisphere of 58/60 participants and in the left hemisphere of 59/60 participants. For our analyses, our inclusion criteria were to include participants who had at least one pimfs component in each hemisphere (N = 57). When both the dorsal and ventral components were present in a given hemisphere, we merged these components into one pimfs label using the FreeSurfer function mris_mergelabels. Thus, our findings are reported for the merged label. This process resulted in 2,157 manually defined sulci.
Distinction among sulcal types
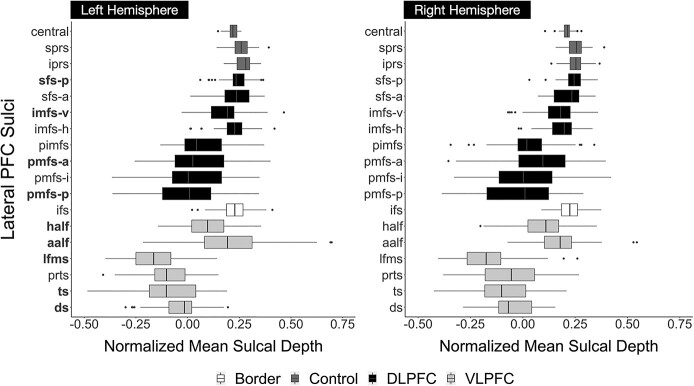
As described in our previous work, as well as classic studies, tertiary sulci are defined as the last sulci to emerge in gestation after the larger and deeper primary and secondary sulci (Cunningham 1892; Retzius 1896; Connolly 1940, 1950; Turner 1948; Bailey et al. 1950; Bailey and von Bonin 1951; Sanides 1964; Chi et al. 1977; Welker 1990; Weiner et al. 2014; Weiner and Zilles 2016; Petrides 2019; Weiner 2019; Miller et al. 2021a, 2021b). Previous studies define primary sulci as sulci that emerge before 32 weeks in gestation, secondary sulci as those emerging between 32 and 36 weeks in gestation, and tertiary sulci as sulci that emerge during and after 36 weeks in gestation (Chi et al. 1977; Connolly 1940, 1950; Cunningham 1892; Miller et al. 2021a, 2021b; Retzius 1896; Turner 1948). Based on these definitions, we consider the cs, sprs, iprs, sfs-p, sfs-a, and ifs as primary sulci, and the pmfs-p, pmfs-i, pmfs-a, pimfs, ds, ts, aalf, half, lfms, and prts as putative tertiary sulci. The definitions of these latter 10 sulci as putative tertiary sulci are further supported by their shallow depth (Fig. 2), which is a defining morphological feature of tertiary sulci.
Fig. 2.
VLPFC sulci are shallower and more variable than DLPFC sulci in a developmental cohort. Comparison of the normalized mean sulcal depths in the left and right hemispheres. Mean sulcal depth at each vertex in standard FreeSurfer units was normalized to the maximum depth within each hemisphere. Boxplots denoted in black represent DLPFC sulci, which are comparatively deeper and exhibit less variability in depth across participants. Boxplots in light gray represent VLPFC sulci, which are comparatively shallower and demonstrate more variability in sulcal depth across participants. Dark gray boxplots represent sulci (cs, sprs, iprs) not included in these regions that are considered control sulci. The ifs (white) demarcates the boundary between DLPFC and VLPFC, and thus is not included in either region. The sulci whose depths predict working memory manipulation—left hemisphere ds, ts, lfms, aalf, half, pmfs-p, pmfs-a, sfs-p, and imfs-v—are bolded on the y-axis.
Apart from these sulci, the question of whether other LPFC sulci should be considered secondary or tertiary is still unresolved. For example, the imfs-v and imfs-h are contemporary labels for classic definitions of sulci commonly labeled as either the frontomarginal and/or middle frontal sulci (Petrides 2019; Miller et al. 2021a, 2021b). When considering classic papers and atlases (Cunningham 1892; Retzius 1896; Connolly 1940; Turner 1948), both the imfs-h and imfs-v appear to be prevalent prior to 32 weeks, which would define them as primary sulci. Yet, additional studies define sulci in this cortical expanse as secondary (Tamraz and Comair 2006).
As in our previous studies (Miller et al. 2021a, 2021b; Voorhies et al. 2021), we consider the imfs-h and imfs-v as primary sulci, but it is possible that future studies will establish them as secondary sulci. Because the definition of primary, secondary, and tertiary sulci remains contentious, we refer to LPFC tertiary sulci explored in the present study as putative. Future research leveraging noninvasive fetal imaging should further improve the distinctions among primary, secondary, and tertiary sulci. Critically, our data-driven approach—and in turn, our findings—are agnostic to these distinctions. That is, the model-based approach adopted here quantitatively determines which sulci best predict WM performance, regardless of their classification.
Characterization of sulcal morphology
For each individual, mean sulcal depth values were extracted for each sulcal label in each hemisphere by intersecting the label file with the .sulc file generated by FS using custom Python code (Miller et al. 2021a; Voorhies et al. 2021). Raw depth metrics (standard FreeSurfer units) were computed in native space from the .sulc file generated in FreeSurfer 6.0.0. To account for differences in cortical depth across individuals and hemispheres, mean sulcal depth of each sulcus is reported as a proportion of maximum depth in each hemisphere (Voorhies et al. 2021). Mean cortical thickness of each sulcus was also considered as an additional metric to examine the extent to which the relationships between sulcal depth and behavior were specific or extended to other morphological features.
Comparison between DLPFC and VLPFC
To compare depth of sulci in the DLPFC and VLPFC, we conducted a two-way (region × hemisphere) repeated measures analysis of variance (rm-ANOVA; Fig. 2). DLPFC and VLPFC mean sulcal depth for each participant was calculated for each sulcus in each region. To assess variability between hemispheres and prefrontal regions, we also conducted the same rm-ANOVA using standard deviation instead of mean sulcal depth. DLPFC and VLPFC standard deviation for each participant was calculated for each sulcus in each region. ANOVAs were computed in R with the aov function.
Relating sulcal morphology and behavior
Model selection
To determine which sulci, if any, were associated with WM performance, we employed a least absolute shrinkage and selection operator (LASSO) regression (Voorhies et al. 2021), separately for each hemisphere. For each task, the depth of all 18 sulci was included as predictors in the model, along with age. Prior to performing this analysis, we ensured that individual variability in depth was not strongly correlated with age (Supplementary Fig. 2).
A LASSO regression is well suited to this situation as it facilitates model selection and increases the generalizability of a model by providing a sparse solution that reduces coefficient values and decreases variance in the model without increasing bias (Heinze et al. 2018). LASSO performs L1 regularization by applying a penalty, or shrinking parameter (α), to the absolute magnitude of the coefficients such that low coefficients are set to zero and eliminated from the model. In this way, LASSO can facilitate data-driven variable selection, leading to simplified models where only the sulci most associated with task performance remain as predictors in the model. This approach improves the interpretability and prediction accuracy of a model while also guarding against overfitting and thus improving its generalizability (Heinze et al. 2018; Ghojogh and Crowley 2019).
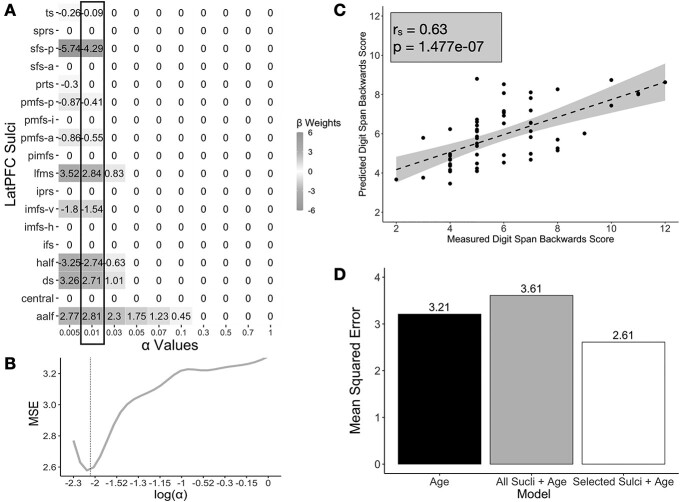
As part of the model selection process, we used cross-validation to optimize the values for the shrinking parameter and we used the GridSearchCV function from the sklearn package to perform an exhaustive search across a range of alpha values. According to convention, we then selected the parameter value that minimized the cross-validated error (LH: a = 0.01; Fig. 3; Heinze et al. 2018) using the following formula:
Fig. 3.
Performance and model fits relating sulcal depth to WM performance. A) Beta-coefficients for each sulcus at a range of shrinking parameter (α) values resulting from the LASSO regression predicting Digits Backward score from sulcal depth in the left hemisphere. Highlighted box indicates coefficients at the chosen alpha-level. B) MSEcv at each alpha-level. We selected the value of α that minimized the cross-validated MSE (dotted line). C) Spearman’s correlation (rs = 0.63) between participants’ actual scores on Digits Backward and the predicted scores resulting from the LOOCV for the best performing model, which had only left-hemisphere LASSO-selected sulci. D) Model comparison of the cross-validated MSEs of a model with age as its only predictor (black), a model with all the left-hemisphere sulci and age as predictors (gray), and a model with sulcal depth of selected sulci in the left hemisphere and age as predictors (white). The model with left-hemisphere sulci selected by the LASSO regression (white) had the lowest cross-validated MSE, performing the best.
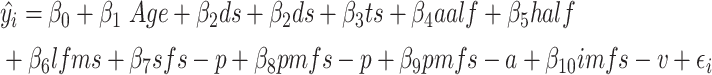 |
(1) |
In addition, we implement the following 2-pronged approach:
Regularization: We use L1 regularization (LASSO regression) as part of our model-selection approach. Not only does this technique provide a data-driven method for model selection, but also regularization is recommended in cases where there are a large number of predictors (X > 10) as this technique guards against overfitting and increases the likelihood that a model will generalize to other datasets. Unlike many techniques that only assess generalizability, L1 regularization actually increases the generalizability of a model by providing a sparse solution that reduces coefficient values and decreases variance in the model without increasing bias (Heinze et al. 2018).
Cross-validation: We used cross-validation in 2 contexts. First, we used it to optimize the shrinking parameter for the LASSO regression. By convention we selected the model parameters that minimized the cross-validated mean squared error (MSE). This optimization procedure was implemented with the GridSearchCV function from sklearn in python. The grid search function uses cross-validation to perform exhaustive search over specified parameters values for an estimator (https://scikit-learn.org/stable/modules/generated/sklearn.model_selection.GridSearchCV.html) Second, all models were fit with standard leave-one-participant-out cross-validation. This allowed us to better assess the generalizability of each model within a sample. In all instances, the reported model fits and coefficients are those from the cross-validated models. All regression models were implemented with the sklearn package in python.
Model comparisons
From the LASSO regression, we identified our model of interest, which included a subset of 9 LH sulci that were strongly associated with verbal WM manipulation. As expected, age was also a strong predictor of task performance. To specifically characterize the relationship between sulcal depth of these 9 sulci and verbal WM manipulation performance, we compared our model of interest with two alternative nested models. All regression models were fit in sklearn with a leave-one-out cross-validation (LOOCV) procedure. First, to verify the results of our feature selection, we compared our simplified model of interest [1] with a full model that included all LH sulci [2]. We focused on LH sulci as our initial model only identified sulci in this hemisphere (Fig. 3).
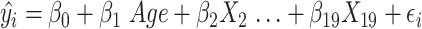 |
(2) |
In the nested full model, x2—x19 represent the sulcal depth of each of the 18 LPFC sulci in one hemisphere, and β2—β19 represent the associated coefficients.
Given that verbal WM manipulation was correlated with age, we compared our model of interest, which included both age and sulcal depth [1], to a model with age as the sole predictor [3]. This nested comparison allowed us to determine whether the sulci in our chosen model explained variance in verbal WM performance not captured by age alone.
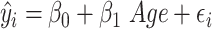 |
(3) |
All models were fit with LOOCV. As these are nested models (the largest model contains all elements in the smaller models), the best fit was determined as the cross-validated model with the lowest cross-validated MSE and the highest R-squared value. Linear models were fit using the SciKit-learn package in Python.
Model specificity
To ascertain whether or not any observed relationship between sulci and task performance generalized to other morphological features, we used mean cortical thickness instead of normalized mean sulcal depth as a predictor of Digit Span task scores. We then compared the fit of the best sulcal depth model with the cortical thickness model using the Akaike Information Criterion (AIC; Akaike 1974). By comparing AIC scores, we could assess the relative performance of the two models. If the ΔAIC is >2, it suggests an interpretable difference between models. If the ΔAIC is >10, it suggests a strong difference between models, with the lower AIC value indicating the preferred model (Wagenmakers and Farrell 2004).
Results
For each participant, cortical surface reconstructions were generated using T1-weighted MPRAGE scans, and 18 LPFC sulci were manually labeled in each hemisphere. Depth and mean cortical thickness were calculated for each sulcus. To account for systematic individual and hemispheric differences in brain size, sulcal depth is calculated as a percentage of maximum depth in each hemisphere. This normalized sulcal depth is reported in standard Freesurfer units. To assess the relation between sulcal anatomy and verbal WM performance, we applied a data-driven approach (Voorhies et al. 2021). Below, we discuss the results of our approach in which we found that (i) VLPFC sulci are shallower and more variable than DLPFC sulci, (ii) sulcal depth is related to verbal WM manipulation, (iii) 9 left-hemisphere LPFC sulci, but no right-hemisphere LPFC sulci defined in the present study, predict manipulation scores, and (iv) these brain-behavior relations are not generalizable, as the cortical thickness of these 9 sulci is not related to verbal WM performance.
Tertiary sulci are consistently identifiable in LPFC of 6–18-year-olds, and VLPFC sulci are shallower and more variable than DLPFC sulci
Seventeen of the 18 LPFC sulci were identifiable in both hemispheres in all participants. Only the pimfs, a tertiary sulcus, was unidentifiable in the right hemisphere for two participants and in the left hemisphere for one participant (Supplementary Fig. 1). A two-way rm-ANOVA was conducted to statistically test for differences between LPFC region (DLPFC, VLPFC) and hemisphere. The rm-ANOVA revealed a main effect of region (F(1,56) = 295.7, P < 0.001), showing that VLPFC sulci were shallower than DLPFC sulci (MVLPFC = −0.01; MDLPFC = 0.12; Fig. 2). However, there was no effect of hemisphere on sulcal depth (F(1,56) = 1.84, P = 0.18) or a hemisphere × region interaction (F(1,56) = 0.26, P = 0.61). To explore the variability between LPFC regions, we repeated this rm-ANOVA substituting mean sulcal depth with standard deviation of sulcal depth. This analysis revealed a main effect of region (F(1,56) = 6.18, P = 0.01), showing that the depths of VLPFC sulci were more variable than the depths of DLPFC sulci (SDVLPFC = 0.18; SDDLFPC = 0.16). We also observed an effect of hemisphere on standard deviation of sulcal depth (F(1,56) = 8.44, P = 0.01), but did not observe a hemisphere × region interaction (F(1,56) = 0.073, P = 0.79).
Sulcal depth is associated with WM manipulation, but not maintenance
To characterize the relationship between sulcal depth and the 2 aspects of WM (manipulation and maintenance), we applied feature selection to determine which sulci, if any, were associated with either maintenance (Digits Forward) or manipulation (Digits Backward). To do so, we implemented a LASSO regression relating the depths of 18 sulci (Fig. 1) as well as age, to scores on the two tasks (Materials and Methods). A LASSO regression not only allows us to select sulci in a data-driven manner, but also improves the generalizability of a model and prevents overfitting, particularly in cases where there are 10 < x < 25 predictors (Heinze et al. 2018).
We assessed the relationship between sulcal depth and maintenance and manipulation separately in each hemisphere. Age was included as an additional predictor in all models. This approach revealed a significant association between normalized mean sulcal depth and Digits Backward score in the left (R2CV = 0.40, MSE = 2.61, a = 0.01), but not the right (R2CV = 0.25, MSE = 3.22, a = 0.1), hemisphere. Neither left nor right hemisphere sulcal depth was related to Digits Forward score (LH: R2CV = 0.26, MSE = 3.27, a = 0.07; RH: R2CV = 0.26, MSE = 3.27, a = 0.07), even though Digits Forward and Backward scores were correlated across participants (r = 0.51, P = 0.001). As predicted, age was also associated with WM performance for both the Digits Backward condition (R2CV = 0.25, MSE = 3.21) and Digits Forward condition (R2CV = 0.26, MSE = 3.21). In addition, there was a positive relationship between task performance and age: The older the participant, the better the performance on Digits Backward (βage = 0.34) and Digits Forward (β age = 0.35) tasks.
The depths of 9 left-hemisphere LPFC sulci predict WM manipulation task performance
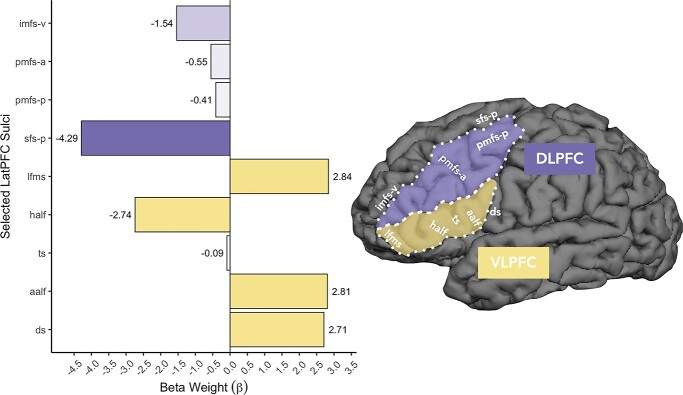
Examining the coefficients of the manipulation models revealed that 9 out of 18 left-hemisphere LPFC sulci, but none of the right hemisphere sulci, were related to performance for the Digits Backward task. In left VLPFC, the ds, ts, lfms, aalf, and half were predictors in the model (Fig. 3A, Fig. 4—Left). In left DLPFC, the sfs-p, imfs-v, pmfs-p, and pmfs-a were additionally predictive (Fig. 3A, Fig. 4—Left). These sulci were selected based on the results of the LASSO regressions. Six of the 9 sulci exhibited negative relationships with verbal WM manipulation performance, whereas three others demonstrated positive relationships with verbal WM manipulation performance (Fig. 3).
Fig. 4.
The relationship among function, sulcal morphology, and WM skills in the developing LPFC. A) Barplot comparing the beta weights for Digits Backward for each of the 9 left-hemisphere sulci identified by the model. Negative and positive beta-coefficients represent negative and positive relationships between sulcal depth and task performance, respectively. All 4 DLPFC (purple) sulci show negative relationships with verbal WM manipulation, whereas 3/5 VLPFC (yellow) sulci show positive relationships with verbal WM manipulation. Darker shades represent stronger beta-coefficients. These values are also shown in Fig. 3A. B) An example cortical surface reconstruction of a left hemisphere in an example 15-year-old (n039t2). Sulcal depth predicted WM skills in the ts, aalf, half, lfms, pmfs-p, pmfs-a, sfs-p, and imfs-v (white labels). The ds, aalf, half, lfms, and imfs-v form the boundaries of the functional clusters preferentially coding the serial order of visual stimuli in WM identified by Amiez and Petrides (2007) in mid-DLPFC and mid-VLPFC and have stronger relationships with verbal WM manipulation than the ts and pmfs-a, which fall within our modified versions of these functional borders in which the present versions extend more posteriorly. Purple: Based on macroanatomical definition of mid-DLPFC in Amiez and Petrides (2007), our DLPFC definition extends more posteriorly and includes sfs-p, pmfs-i, and pmfs-p. Yellow: Based on macroanatomical definition of mid-VLPFC in Amiez and Petrides (2007), our VLPFC definition also includes the ds. See Supplementary Fig. 3 for comparisons of sulcal definitions with Amiez and Petrides (2007).
To further examine the relationship between the depth of these specific sulci and verbal WM manipulation, we used the partial model derived from our LASSO regression to predict performance. All models were fit with LOOCV. The partial model only used the mean sulcal depths of left-hemisphere ds, ts, lfms, aalf, half, sfs-p, imfs-v, pmfs-p, and pmfs-a as predictors of Digits Backward scores in the LOOCV linear regression. Age was also included as a predictor in the model. The results of the LOOCV linear regression confirmed that the partial model with these 9 sulci significantly predicted Digits Backward scores (rs = 0.63, P = 0.0000001; Fig. 3C). When compared with an alternative nested cross-validated model that included all the LPFC sulci in the left hemisphere, we found that the addition of the other 9 sulci did not improve the fit of the model (R2CV = 0.16, MSEcv = 3.61; Fig. 3D). This comparison was consistent with the predictions of the LASSO regression.
To ascertain that the relationship between sulcal depth and verbal WM manipulation performance was not driven by age (see Supplementary Fig. 2 for relationship between age and depth of each sulcus), we additionally compared the left-hemisphere partial model to another alternative nested cross-validated model with age as the sole predictor (R2CV = 0.26, MSEcv = 3.21; Fig. 3D). Age at the time of assessment was positively correlated with scores on the Digits Backward task (r = 0.57, P = 0.001). However, when compared with the age model, the left-hemisphere partial model (R2CV = 0.40, MSEcv = 2.61) showed a better fit. Thus, age alone did not explain the results of the partial model. The inclusion of the selected sulci improved prediction of Digits Backward scores above and beyond age.
Sulcal depth, not sulcal cortical thickness, is associated with verbal WM performance
To assess whether the association between sulcal depth and verbal WM manipulation performance extended to other morphological features of the sulci included in the partial models, we also used a LASSO approach with mean cortical thickness of each sulcus as the predictor. Again, age was included as an additional predictor in the left-hemisphere partial models. We found a slight positive relationship between mean sulcal cortical thickness of the 9 left-hemisphere sulci and Digits Backward scores (R2CV = 0.04, MSEcv = 4.12). Nevertheless, the cross-validated sulcal cortical thickness model did not perform better than age in predicting Digits Backward scores. In addition, sulcal depth was a better predictor of verbal WM manipulation than was sulcal cortical thickness, as assessed by the Akaike Information Criterion (AICCorticalThickness = 94.97, AICSulcalDepth = 67.49, △AIC = 27.48; Akaike 1974). If the ∆AIC is >2, it suggests an interpretable difference between models; the lower AIC value indicates the preferred model (Burnham and Anderson 2004; Wagenmakers and Farrell 2004).
Discussion
To our knowledge, this is the first study to examine the relationship between DLPFC and VLPFC tertiary sulcal morphology and verbal WM performance in a developmental cohort. Implementing a data-driven approach with cross-validation, we showed that there is a relationship between sulcal depth and verbal WM performance on the widely used Digit Span task: The depth of 9 left-hemisphere sulci predicted verbal WM performance on the challenging Digits Span Backwards task. In contrast, none of the sulci predicted pure WM maintenance, as measured by the Digits Span Forwards task.
As noted previously, our study addressed three main questions. First, we sought to test for a relationship between verbal WM and mean depth of LPFC sulci. Our results support this hypothesis, showing an anatomical-behavioral relationship for numerous LPFC sulci. Second, we asked whether any such relationships differed as a function of sulcal type, hemisphere, and/or task demands. We found relationships for both shallow/tertiary and deep/non-tertiary sulci. However, there was a hemispheric effect: All of the sulci implicated in verbal WM were localized in the left, language-dominant hemisphere. We also found an effect of task demand: We only observed relationships between sulcal depth and performance when the task required manipulation of items in WM. Third, we asked whether we could construct a model to predict an individual’s verbal WM task score from sulcal depth. Indeed, using a LASSO regression to select the sulci most related to performance and a LOOCV linear regression to predict scores on the task, we were able to construct a model including age and the 9 left LPFC sulci that better predicted scores compared to separate models with either all LPFC sulci or age alone.
The sulci predicting verbal WM manipulation included 7 out of 10 of the putative tertiary sulci labeled in this study, as well as 2 of the 8 primary sulci. Although greater sulcal depth predicted better performance for 3 sulci, shallower sulcal depth predicted better performance for 6 sulci. In the sections below, we discuss (i) the location of the implicated sulci relative to functional and anatomical features of LPFC, (ii) implications of hemispheric and task dissociations, (iii) the fact that relationships to WM performance were not observed for sulcal cortical thickness, (iv) possible mechanism(s) linking sulcal depth and human behavior, and (v) future directions and limitations of our study.
Location of the implicated sulci relative to functional and anatomical features of LPFC
The locations of a subset of the 9 sulci in the left hemisphere identified by our model are approximately aligned with the functional definitions of mid-DLPFC and mid-VLPFC as identified by Amiez and Petrides (2007; Fig. 4). To include all sulci within LPFC, our DLPFC and VLPFC definitions extended more posteriorly and included sfs-p, pmfs-i, pmfs-p, and the ds, three of which were chosen by our model-based approach (see Supplementary Fig. 3 for a direct comparison between the sulcal definitions in Amiez and Petrides (2007) and the sulcal definitions in the present study). Relative to the functional demarcations of mid-DLPFC and mid-VLPFC in Amiez and Petrides (2007), the aalf, half, lfms, and imfs-v form the anterior, inferior, and superior boundaries of the functional clusters preferentially coding the serial order of visual stimuli in WM. These boundary sulci also have stronger relationships with verbal WM manipulation than the ts and pmfs-a, which fall within these functional borders. Thus, a subset of sulci identified by our model-based approach could serve as potential landmarks—a hypothesis that can be examined in future studies.
Consistent with this idea, some of the sulci identified by our model-based approach are located in different cytoarchitectonic and multimodal areas associated with different cognitive functions (Supplementary Fig. 3), as well as embedded in certain locations within anatomical and functional LPFC gradients. For instance, the classic mid-DLPFC region associated with WM contains areas 9/46 and 46 (Petrides and Pandya, 1999); pmfs-i/a is located within the former, and imfs-h/pimfs-d in the latter. In addition to considering the dorsal–ventral axis of LPFC, it will be important to further explore dissociations between sulci along the anterior–posterior axis. Indeed, recent work in adults shows that LPFC tertiary sulci measured here have different profiles of functional connectivity and are located within a broader myelination gradient, as measured by the T1/T2 ratio, with the highest content at the central sulcus posteriorly and lowest content anteriorly in frontopolar cortex (Miller et al. 2021a, 2021b). Interestingly, previous work also shows that this T1/T2 gradient changes with age along an anterior–posterior gradient in LPFC (Norbom et al. 2020, 2021) into frontopolar cortex, and shows an interaction between age and sex (see also Paus et al. 2008, 2010, 2017). Thus, future studies could implement a similar model-based approach relating sulcal morphology and cognition as implemented here, as well as incorporate additional features into the model, such as T1/T2 ratio or quantitative MRI measures.
Implications of hemispheric and task dissociations
With regards to the set of 9 sulci implicated in WM, it is notable that, first, all were in the left hemisphere and, second, all were related to Digits Backward but not Forward task performance. These patterns reinforce emerging views of the role of LPFC in WM.
The laterality effect is consistent with extensive neuropsychological and neuroimaging evidence that verbal tasks, including verbal WM tasks, predominantly implicate the left hemisphere (e.g. Black 1986a, 1986b; Fiez 1997; Smith and Jonides 1998; Wagner et al. 2001; Laures-Gore et al. 2011). Most directly related to the present findings, a patient study involving the same span tasks as in the present work showed that left, but not right, DLPFC lesions were associated with deficits in verbal WM manipulation, as measured by the Backwards span task (Barbey et al. 2013).
The task effect—that is, the fact that LPFC sulcal depth was related to Digits Backward but not Forward span task performance—is also consistent with this prior patient study: Barbey and colleagues found that DLPFC lesions were associated with deficits on the Digits Backward but not Forward span task. Moreover, Baldo and Dronkers (2006) showed that left inferior parietal—but not left VLPFC—lesions were associated with impaired Digits Forward span task performance, consistent with the purported role of left inferior parietal cortex in phonological storage (Smith and Jonides 1998; see Fiez 2001).
Indeed, the present findings complement a mounting body of evidence that LPFC’s involvement in WM is most critical on tasks that require a high level of active processing of temporarily activated memory representations (Nee and D'Esposito 2018), such as the Backwards digit span task. As such, performance on a pure maintenance task like the Digits Forward span task could be related to the sulcal morphology of regions outside LPFC—in particular, left inferior parietal cortex. Together, these findings suggest that individual differences in sulcal development impact the functional organization of LPFC-dependent cognitive function identified here and in previous work (Im et al. 2008; Garrison et al. 2015; Voorhies et al. 2021).
Relationships to WM performance were not observed for sulcal cortical thickness
We observed no relationship between the thickness of sulcal gray matter and WM (Krogsrud et al. 2021), which is consistent with our previous work looking at the relationship between LPFC sulcal morphology and reasoning skills in the same cohort (Voorhies et al. 2021). This contrasts with prior studies showing a negative relation between cortical thickness and WM; however, it should be noted that the current study focused on sulcal thickness specifically, rather than across large expanses of cortex including both gyri and sulci. In addition, cortical thinning likely reflects both gray matter thinning and the myelination of fibers extending into the cortical ribbon (Paus 2005). Consistent with this idea, Natu et al. (2019) showed that myelination is a key contributor to cortical thinning in visual cortex during childhood. We also note that although previous research shows age-related changes for anatomical features such as myelin content as measured by the T1/T2 ratio in LPFC extending into frontopolar cortex (Paus et al. 2008, 2010, 2017; Norbom et al. 2020, 2021), we do not see a clear relationship between sulcal depth and age in our developmental sample (Supplementary Fig. 2). Thus, as expanded further in the next section, future research examining the relations between sulcal depth and white matter is warranted.
Possible mechanism(s) linking sulcal depth and human behavior: short-range connections and local gyrification?
Of the 9 left-hemisphere sulci whose depths predicted verbal WM performance, 7 were putative tertiary sulci, consistent with Sanides’ theory that tertiary sulcal anatomy is relevant for higher cognition (1962, 1964). Specifically, mirroring the fact that primary sulci, which emerge early in gestation, serve as landmarks in primary sensory cortices, Sanides posited that tertiary sulci, which emerge late in gestation, serve as landmarks in association cortices, which also show a protracted development. He further proposed that the late emergence and continued postnatal morphological development of tertiary sulci is likely related to cognitive skills associated with LPFC that also show a protracted development. Although our results support this hypothesis by showing that tertiary sulci are behaviorally meaningful, we did not find that only tertiary sulci were linked to cognitive performance. This fits with previous findings that also showed relationships between non-tertiary sulcal morphology in other cortical locations and cognitive performance in children (Roell et al. 2021).
We have recently proposed that deeper tertiary sulci in LPFC could be indicative of short-range connections that function to pull regions closer together and, in turn, are associated with greater efficiency of neural processing by decreasing the distance between LPFC regions (Voorhies et al. 2021). This heightened neural efficiency could manifest as improved behavioral performance. This mechanistic hypothesis was proposed based on recent and classic findings showing that (i) short white-matter fibers extend from the deepest points of sulci into the white matter (Reveley et al. 2015) and (ii) there is a relationship between tertiary sulci in LPFC and myelination (Sanides 1962; Miller et al. 2021a; 2021b). The present findings build on this proposal by showing that a combination of shallower and deeper sulci predicts verbal WM performance.
Because in some cases we found a negative rather than positive relation between sulcal depth and cognitive performance, we speculate that additional anatomical mechanisms, such as those related to the development of neighboring sulci or white matter tracts, are likely at play. For instance, several researchers (Connolly 1950; Armstrong et al. 1995; Zilles et al. 2013) qualitatively noted that the sizes and depths of sulci seemingly counterbalance those of nearby sulcal neighbors. Thus, a shallow, short sulcus would compensate for a particularly long and deep nearby sulcus, rendering the overall degree of cortical folding within a given region approximately equal (Connolly 1950; Armstrong et al. 1995; Zilles et al. 2013).
Given this hypothesis, we propose that a relatively deeper tertiary sulcus, which may have stronger short-range white matter connections as proposed previously (Voorhies et al. 2021), may be close to sulci that are relatively shallower, thereby preserving the degree of local cortical folding. This proposal builds on a recent modification of the compensation theory of cortical folding that proposes to also incorporate local morphological features (Natu et al. 2020). Altogether, our findings begin to build a multimodal mechanistic neuroanatomical understanding underlying the complex relationship between sulcal depth and cognition relative to other anatomical features, which importantly makes explicit, testable predictions for future studies.
Future directions and limitations
Although our sample size reflects those of other studies of this nature (Weiner et al. 2014, 2018; Sprung-Much and Petrides 2018, 2020; Borne et al. 2020; Miller et al. 2021a, 2021b; Eichert et al. 2021; Voorhies et al. 2021), the main limitation of our study is the labor-intensive process of manually identifying sulci, which limits the number of participants. Current methods implement deep learning algorithms to automatically identify primary and secondary sulci in LPFC (Hao et al. 2020). Thus, modifications to these algorithms to include tertiary sulci would make it possible to expand sample sizes in future studies examining the relationship between the morphology of tertiary sulci and cognition. Ongoing work is already underway to develop deep learning algorithms to accurately define tertiary sulci automatically in individual participants, and initial results are promising (Borne et al. 2020; Lyu et al. 2021).
In addition, verbal WM depends on a distributed neural system; thus, it is likely that verbal WM performance is also related to sulcal variation in other regions implicated in WM, such as lateral parietal cortex (Goldman-Rakic 1990; Smith and Jonides 1998; Crone et al. 2006; Klingberg 2006; Tamnes et al. 2013). Furthermore, although it is common to relate brain structure to performance on individual tasks, as we have done here, the limited number of trials administered during the Digit Span task restricts our ability to investigate verbal WM performance in depth. Thus, the use of a latent construct or composite score derived from multiple verbal WM measures would permit us to further assess the generalizability of our cross-validated results (see Bollen 2002).
To explore why various sulci showed opposite relationships between depth and performance, future work should also examine relationships between the development of neighboring sulci, as well as the underlying white matter tracts linking these sulci. The former will allow us to understand if compensation, whereby shallower or shorter sulci balance nearby longer or deeper sulci, is at play. The latter will reveal if sulcal depth is linked to white matter connections and increased neural efficiency, which may be related to individual differences in cognitive performance.
Finally, future studies involving larger sample sizes should also consider how other variables like demographics, genetics, and early environment also contribute to this relationship between sulcal depth and cognition. Semi-automation of tertiary sulcal definitions further render the feasibility of larger-scale investigations (Borne et al. 2020; Lyu et al. 2021).
Conclusion
These findings highlight the behavioral significance of individual variability in sulcal morphology. Some of these sulci may serve as boundaries for functional regions engaged in verbal WM manipulation. These results begin to shed light on the complex relationship among sulcal morphology in LPFC, parcellations of LPFC, and verbal WM skills in children and adolescents. More broadly, these and our prior findings based on a largely overlapping pediatric MRI dataset (Voorhies et al. 2021) contribute to an increasing body of work in adults that also empirically support Sanides’ hypothesis that tertiary sulci serve as functional and cognitive landmarks in association cortices. Taken as a whole, this emerging body of research indicates the importance of studying tertiary sulci to better understand the relationships among brain structure, brain function, and behavior.
Supplementary Material
Acknowledgments
We thank Ishana Raghuram, Chahat Mittal, and Anmol Gill for assistance with sulcal labeling and former members of the Bunge laboratory for assistance with data collection, and the families who participated in the study.
Contributor Information
Jewelia K Yao, Princeton Neuroscience Institute, Princeton University, Washington Rd, Princeton, NJ 08540, United States.
Willa I Voorhies, Department of Psychology, University of California, Berkeley, 2121 Berkeley Way, Berkeley, CA 94720, United States.
Jacob A Miller, Helen Wills Neuroscience Institute, University of California, Berkeley, 175 Li Ka Shing Center, Berkeley, CA 94720, United States.
Silvia A Bunge, Department of Psychology, University of California, Berkeley, 2121 Berkeley Way, Berkeley, CA 94720, United States; Helen Wills Neuroscience Institute, University of California, Berkeley, 175 Li Ka Shing Center, Berkeley, CA 94720, United States.
Kevin S Weiner, Department of Psychology, University of California, Berkeley, 2121 Berkeley Way, Berkeley, CA 94720, United States; Helen Wills Neuroscience Institute, University of California, Berkeley, 175 Li Ka Shing Center, Berkeley, CA 94720, United States.
Funding
This research was supported by a T32 HWNI training grant and an NSF-GRFP fellowship (to W.I.V.). Funding for the original data collection and curation was provided by the National Institute of Neurological Disorders and Stroke (R01 NS057156 to S.A.B.) and the National Science Foundation (BCS1558585 to S.A.B.). Data analyses were supported by National Institute of Child Health and Human Development (R21HD100858 to K.S.W. and S.A.B.) and the National Science Foundation (CAREER 2042251 to K.S.W.).
Conflict of interest statement. The authors declare no competing financial or potential conflicts of interests.
References
- Akaike H. 1974. A new look at the statistical model identification. In: Parzen E, Tanabe K, Kitagawa G. (eds) Selected papers of Hirotugu Akaike. Springer series in statistics (perspectives in statistics). Springer, New York (NY). 10.1007/978-1-4612-1694-0_16. [DOI] [Google Scholar]
- Amiez C, Petrides M. Selective involvement of mid-dorsolateral prefrontal cortex in the coding of the serial order of visual stimuli in working memory. Proc Natl Acad Sci U S A. 2007:104:13786–13791. 10.1073/pnas.0706220104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Wilson CRE, ProcykE. Variations of cingulate sulcal organization and link with cognitive performance. Nature Sci Rep. 2018:8(13988). 10.1038/s41598-018-32088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cereb Cortex. 1995:5:56–63. 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G. Working memory. Psychol Learn Motiv. 1974:8:47–89. 10.1016/s0079-7421(08)60452-1. [DOI] [Google Scholar]
- Badre D. Cognitive control, hierarchy and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008:12:193–200. 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Bailey P, von Bonin GV. The isocortex of man. Urbana (IL): University of Illinois Press; 1951. [Google Scholar]
- Bailey P, von Bonin GV, McCulloch WS. The isocortex of the chimpanzee. Urbana, IL: University of Illinois Press; 1950. [Google Scholar]
- Baldo JV, Dronkers NF. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology. 2006:20:529–538. 10.1037/0894-4105.20.5.529. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Koenigs M, Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex. 2013:49:1195–1205. 10.1016/j.cortex.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathelt J, Gathercole SE, Johnson A, Astle DE. Differences in brain morphology and working memory capacity across childhood. Dev Sci. 2018:21:e12579. 10.1111/desc.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black FW. Digit repetition in brain-damaged adults: clinical and theoretical implications. J Clin Psychol. 1986a:42:770–782 . [DOI] [PubMed] [Google Scholar]
- Black FW. Neuroanatomic and neuropsychological correlates of digit span performance by brain-damaged adults. Percept Mot Skills. 1986b:63:815–822. 10.2466/pms.1986.63.2.815. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Nomura EM, Gratton C, D’Esposito M. Lateral prefrontal cortex is organized into parallel dorsal and ventral streams along the rostro-caudal axis. Cereb Cortex. 2013:23:2457–2466. 10.1093/cercor/bhs223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen KA. Latent variables in psychology and the social sciences. Annu Rev of Psychol. 2002:53:605–634. 10.1146/annurev.psych.53.100901.135239. [DOI] [PubMed] [Google Scholar]
- Borne L, Riviere D, Mancip M, Mangi JF. Automatic labeling of cortical sulci using patch- or CNN-based segmentation techniques combined with bottom-up geometric constraints. Med Image Anal. 2020:62:101651. 10.1016/j.media.2020.101651. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Curr Opin Neurobiol. 2007:17:243–250. 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res. 2004:33:261–304. 10.1177/0049124104268644. [DOI] [Google Scholar]
- Cachia A, Roell M, Mangin JF, Zhong YS, Jobert A, Braga L, Houde O, Dehaene S, Borst G. How interindividual differences in brain anatomy shape reading accuracy. Brain Struct Funct. 2018:223:701–712. 10.1007/s00429-017-1516-x. [DOI] [PubMed] [Google Scholar]
- Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol. 1977:1:86–93. 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- Chung YS, Hyatt CJ, Stevens MC. Adolescent maturation of the relationship between cortical gyrification and cognitive ability. NeuroImage. 2017:158:319–331. 10.1016/j.neuroimage.2017.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CJ. Development of the cerebral sulci. Am J Phys Anthropol. 1940:26:113–149. 10.1002/ajpa.1330260125. [DOI] [Google Scholar]
- Connolly CJ. External morphology of the primate brain. Springfield (IL): Charles C. Thomas; 1950. [Google Scholar]
- Cowan N. Working memory maturation: can we get at the essence of cognitive growth. Perspect Psychol Sci. 2016:11:239–264. 10.1177/1745691615621279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci U S A. 2006:103:9315–9320. 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D. Contribution to the surface anatomy of the cerebral hemispheres. Dublin (Ireland): Dublin R Irish Acad; 1892. [Google Scholar]
- D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999:41:66–86. 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. NeuroImage. 1999:9:179–194. 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Darki F, Klingberg T. The role of fronto-parietal and fronto-striatal networks in the development of working memory: a longitudinal study. Cereb Cortex. 2015:25:1587–1595. 10.1093/cercor/bht352. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Fenstermacher E, Salat DH, Wolk DA, Maguire RP, Desikan R, Pacheco J, Quinn BT, Van der Kouwe A, Greve DN, et al. Detection of cortical thickness correlates of cognitive performance. NeuroImage. 2008:39:10–18. 10.1016/j.neuroimage.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000:23:475–483. 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Eberstaller O. Das stirnhirn. Vienna: Urban & Schwarzenberg; 1890. [Google Scholar]
- Eichert N, Watkins KE, Mars RB, Petrides M. Morphological and functional variability in central and subcentral motor cortex of the human brain. Brain Struct Funct. 2021:226:263–279. 10.1007/s00429-020-02180-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA. Phonology, semantics, and the role of the left inferior prefrontal cortex. Hum Brain Mapp. 1997:5:79–83. . [DOI] [PubMed] [Google Scholar]
- Fiez JA. Bridging the gap between neuroimaging and neuropsychology: using working memory as a case-study. J Clin Exp Neuropsychol. 2001:23:19–31. 10.1076/jcen.23.1.19.1221. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Petersen SE. A positron emission tomography study of the short-term maintenance of verbal information. J Neurosci. 1996:16:808–822. 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. Upper processing stages of the perception-action cycle. Trends Cogn Sci. 2004:8:143–145. 10.1016/j.tics.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Garrison JR, Fernyhough C, McCarthy-Jones S, Haggard M, The Australian Schizophrenia Research Bank, Simons JS. Paracingulate sulcus morphology is associated with hallucinations in the human brain. Nat Commun. 2015:6:8956. 10.1038/ncomms9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The structure of working memory from 4 to 15 years of age. Dev Psychol. 2004:40:177–190. 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Ghojogh B, Crowley M. The theory behind overfitting, cross validation, regularization, bagging, and boosting: tutorial. Preprint at. 2019: https://arxiv.org/abs/1905.12787. [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016:536:171–178. 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Dev. 1987:58:601–622. 10.2307/1130201. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Brain Res. 1990:85:325–335. 10.1016/s0079-6123(08)62688-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995:14:477–465. 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gregory MD, Kippenham JS, Dickinson D, Carrasco J, Mattay VS, Weinberger DR, Berman KF. Regional variation in brain gyrification are associated with general cognitive ability in humans. Curr Biol. 2016:26:1301–1305. 10.1016/j.cub.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Bao S, Tang Y, Riqiang G, Parvathaneni P, Miller JA, Voorhies W, Yao J, Bunge SA, Weiner KS, Landman BA, Lyu I. 2020. Automatic labeling of cortical sulci using spherical convolutional neural networks in a developmental cohort. In: IEEE international symposium on biomedical imaging (ISBI) Iowa City (IA). 10.1109/isbi45749.2020.9098414. [DOI] [PMC free article] [PubMed]
- Heinze G, Wallisch C, Dunkler D. Variable selection - a review and recommendations for the practicing statistician. Biom J. 2018:60:431–449. 10.1002/bimj.201700067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Lee JM, Seo SW, Kim SH, Kim SI, Na DL. Sulcal morphology changes and their relationship with cortical thickness and gyral white matter volume in mild cognitive impairment and Alzheimer’s disease. NeuroImage. 2008:43:103–113. 10.1016/j.neuroimage.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006: 44:2171–2177. 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003:302:1181–1185. 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Krogsrud SK, Mowinckel AM, Sederevicius D, Vidal-Piñeiro D, Amlien IK, Wang Y, et al. Relationships between apparent cortical thickness and working memory across the lifespan-effects of genetics and socioeconomic status. Dev Cogn Neurosci. 2021:51:100997. 10.1016/j.dcn.2021.100997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laures-Gore J, Marshall RS, Verner E. Performance of individuals with left hemisphere stroke and aphasia and individuals with right brain damage on forward and backward digit span tasks. Aphasiology. 2011:25:43–56. 10.1080/02687031003714426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Qin W, Zhang Y, Jiang T, Yu C. The neuronal correlates of digits backward are revealed by voxel-based morphometry and resting-state functional connectivity analyses. PLoS One. 2012:7:e31877. 10.1371/journal.pone.0031877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Persem A, Verhagen L, Amiez C, Petrides M, Sallet J. The human ventromedial prefrontal cortex: sulcal morphology and its influence on functional organization. J Neurosci. 2019:39:3627–3639. 10.1523/JNEUROSCI.2060-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Leonard C, Thompson P, Kan E, Jolley J, Welcome SE, Toga AW, Sowell E. Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: a longitudinal MRI analysis. Cereb Cortex. 2007:17:1092–1099. 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- Lyu I, Bao S, Hao L, Yao J, Miller JA, Voorhies W, Taylor WD, Bunge SA, Weiner KS, Landman BA. Labeling lateral prefrontal sulci using spherical data augmentation and context-aware training. NeuroImage. 2021:229:117758. 10.1016/j.neuroimage.2021.117758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JA, Voorhies WI, Lurie DJ, D’Esposito M, Weiner KS. Overlooked tertiary sulci serve as a meso-scale link between microstructural and functional properties of human lateral prefrontal cortex. J Neurosci. 2021a:41:2229–2244. 10.1523/JNEUROSCI.2362-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JA, D’Esposito MA, Weiner KS. Using tertiary sulci to map the ‘cognitive globe’ of prefrontal cortex. J Cogn Neurosci. 2021b:3:1–18. 10.1162/jocn_a_01696. [DOI] [PubMed] [Google Scholar]
- Natu VS, Arcaro MJ, Barnett MA, Gomez J, Livingstone M, Grill-Spector K, Weiner KS. Sulcal depth in the medial ventral temporal cortex predicts the location of a place-selective region in macaques, children, and adults. Cereb Cortex. 2020:31:48–61. 10.1101/2020.01.27.921346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natu VS, Gomez J, Barnett M, Jeska B, Kirilina E, Jaeger C, Zhen, Z, Cox S, Weiner KS, Weiskopf N, Grill-Spector K. Apparent thinning of human visual cortex during childhood is associated with myelination. Proc Natl Acad Sci USA. 2019:116:20750–20759. 10.1073/pnas.1904931116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, D'Esposito M. The representational basis of working memory. Curr Top Behav Neurosci. 2018:37:213–230. 10.1007/7854_2016_456. [DOI] [PubMed] [Google Scholar]
- Norbom LB, Rokicki J, Alnæs D, Kaufmann T, Doan NT, Andreassen OA, Westlye LT, Tamnes CK. Maturation of cortical microstructure and cognitive development in childhood and adolescence: A T1W/T2W ratio mri study. Hum Brain Mapp. 2020:41:4676–4690. 10.1002/hbm.25149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbom LB, Ferschmann L, Parker N, Agartz I, Andreassen OA, Paus T, Westlye LT, Tamnes CK. New insights into the dynamic development of the cerebral cortex in childhood and adolescence: Integrating macro- and microstructural MRI findings. Prog Neurobiol. 2021:204:102109. 10.1016/j.pneurobio.2021.102109. [DOI] [PubMed] [Google Scholar]
- Øtsby Y, Tamnes CK, Fjell AM, Walhovd KB. Morphometry and connectivity of the fronto-parietal verbal working memory network in development. Neuropsychologia. 2011:49:3854–3862. 10.1016/j.neuropsychologia.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Owen AM, Evans AC, Petrides M. Evidence for the two-stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cereb Cortex. 1996:6:31–38. 10.1093/cercor/6.1.31. [DOI] [PubMed] [Google Scholar]
- Passingham RE, Wise SP. The neurobiology of the prefrontal cortex: anatomy, evolution, and the origin of insight. Oxford (United Kingdom): Oxford University Press; 2012. [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RSJ. The neural correlates of the verbal component of working memory. Nature. 1993:362:342–345. 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005:9:60–68. 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Paus T, Otaky N, Caramanos Z, Macdonald D, Zijdenbos A, Davirro D, Evans AC. In vivo morphometry of the intrasulcal gray matter in the human cingulate, paracingulate, and superior-rostral sulci: Hemispheric asymmetries, gender differences and probability maps. J Comp Neurol. 1996:376:664–673. . [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008:9:947–957. 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Nawaz-Khan I, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Susman E, Veillette S, Pausova Z. Sexual dimorphism in the adolescent brain: Role of testosterone and androgen receptor in global and local volumes of grey and white matter. Horm Behav. 2010:57:63–75. 10.1016/j.yhbeh.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Paus T, Wong APY, Syme C, Pausova Z. Sex differences in the adolescent brain and body: findings from the Saguenay Youth Study. J Neurosci. 2017:95:362–370. 10.1002/jnr.23825. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond Ser B Biol Sci. 2005:360:781–795. 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Atlas of the morphology of the human cerebral cortex on the average MNI brain. London (United Kingdom): Elsevier Academic Press; 2019. [Google Scholar]
- Petrides M, Alivisatos B, Meyer E, Evans AC. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci U S A. 1993:90:878–882. 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retzius G. Das Menschenhirn. Stockholm (Sweden): Norstedt and Soener; 1896. [Google Scholar]
- Reveley C, Seth AK, Pierpaoli C, Silva AC, Yu D, Saunders RC, Leopold DA, Ye FQ. Superficial white matter fiber systems impede detection of long-range cortical connections in diffusion MR tractography. Proc Natl Acad Sci U S A. 2015:112:E2820–E2828. 10.1073/pnas.1418198112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roell M, Cachia A, Matejko AA, Houdé O, Ansari D, Borst G. Sulcation of the intraparietal sulcus is related to symbolic but not non-symbolic number skills. Dev Cogn Neurosci. 2021:51:100998. 10.1016/j.dcn.2021.100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. Prefrontal interactions reflect future task operations. Nat Neurosci. 2003:6:75–81. 10.1038/nn987. [DOI] [PubMed] [Google Scholar]
- Sanides F. Architectonics of the human frontal lobe of the brain. With a demonstration of the principles of its formation as a reflection of phylogenetic differentiation of the cerebral cortex. Monographien aus dem Gesamtbiete der Neurologie und Psychiatrie. 1962:98:1–201. [PubMed] [Google Scholar]
- Sanides F. Structure and function of the human frontal lobe. Neuropsycholgia. 1964:2:209–219. [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006:440:676–679. 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Natl Acad Sci U S A. 1998:95:12061–12068. 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprung-Much T, Petrides M. Morphological patterns and spatial probability maps of two defining sulci of the posterior ventrolateral frontal cortex of the human brain: the sulcus diagonalis and the anterior ascending ramus of the lateral fissure. Brain Struct Funct. 2018:223:4125–4152. 10.1007/s00429-018-1733-y. [DOI] [PubMed] [Google Scholar]
- Sprung-Much T, Petrides M. Morphology and spatial probability maps of horizontal ascending ramus of the lateral fissure. Cereb Cortex. 2020:30:1586–1602. 10.1093/cercor/bhz189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Knight RT. Principles of frontal lobe functions. 2nd ed. New York (NY): Oxford University Press; 2002. [Google Scholar]
- Tamnes CK, Walhovd KB, Grydeland H, Holland D, Øtsby Y, Dale AM, Fjell AM. Longitudinal working memory development is related to structural maturation of frontal and parietal cortices. J Cogn Neurosci. 2013:25:1611–1623. 10.1162/jocn_a_00434. [DOI] [PubMed] [Google Scholar]
- Tamraz J, Comair Y. Atlas of regional anatomy of the brain using MRI: with functional correlations. Berlin, Germany: Springer-Verlag; 2006. [Google Scholar]
- Tissier C, Linzarini A, Allaire-Duquette G, Mevel K, Poirel N, Dollfus S, Etard O, Orliac F, Peyrin C, Charron S, et al. Sulcal polymorphisms of the IFC and ACC contribute to inhibitory control variability in children and adults. eNeuro. 2018:5:ENEUROe0197-17.2018 10.1523/ENEURO.0197-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner OA. 1948. Growth and development of the cerebral cortical pattern in man. Arch Neurol Psychiatr 59:1–12. 10.1001/archneurpsyc.1948.02300360011001. [DOI] [PubMed] [Google Scholar]
- Voorhies WI, Miller JA, Yao JK, Bunge SA, Weiner KS. Cognitive insights from evolutionarily new brain structures in prefrontal cortex. Nat Commun. 2021:12:5122. 10.1038/s41467-021-25162-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers EJ, Farrell S. AIC model selection using Akaike weights. Psychon Bull Rev. 2004:11:192–196. 10.3758/BF03206482. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL. Prefrontal contributions to executive control: Fmri evidence for functional distinctions within lateral prefrontal cortex. NeuroImage. 2001:14:1337–1347. 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale of children. Fourth ed. WISC-IV; 1974. [Google Scholar]
- Weiner KS. The mid-fusiform sulcus (sulcus sagittalis gyri fusiformis). Anat Rec. 2019:302:1491–1503. 10.1002/ar.24041. [DOI] [PubMed] [Google Scholar]
- Weiner KS, Zilles K. The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia. 2016:83:48–62. 10.1016/j.neuropsychologia.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner KS, Golarai G, Caspers J, Chuapoco MR, Hartmut M, Zilles K, Amunts K, Grill-Spector K. The mid-fusiform sulcus: a landmark identifying both cytoarchitectonic and functional divisions of human ventral temporal cortex. NeuroImage. 2014:84:453–465. 10.1016/j.neuroimage.2013.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner KS, Natu VS, Grill-Spector K. On object selectivity and the anatomy of the human fusiform gyrus. NeuroImage. 2018:173:604–609. 10.1016/j.neuroimage.2018.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker W. Why does cerebral cortex fissure and fold? A review of determinants of gyri and sulci. Cereb Cortex 8b:3 -136. 1990. 10.1007/978-1-4615-3824-0_1. [DOI] [Google Scholar]
- Wendelken C, Ferrer E, Whitaker KJ, Bunge SA. Fronto-parietal network reconfiguration supports the development of reasoning ability. Cereb Cortex. 2016:26:2178–2190. 10.1093/cercor/bhv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C, Ferrer E, Ghetti S, Bailey SK, Cutting L, Bunge SA. Frontoparietal structural connectivity in childhood predicts development of functional connectivity and reasoning ability: a large-scale longitudinal investigation. J Neurosci. 2017:37:8549–8558. 10.1523/JNEUROSCI.3726-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Su S, Schmidt M, Kao CY, Sapiro G. The development of gyrification in childhood and adolescence. Brain Cogn. 2010:72:36–45. 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Ben-Shachar M, Wandell BA. Development of white matter and reading skills. Proc Natl Acad Sci U S A. 2012:109:E3045–E3053. 10.1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Palomero-Gallagher M, Amunts K. Development of cortical folding during evolution and ontogeny. Trends Neurosci. 2013:36:275–284. 10.1016/j.tins.2013.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.