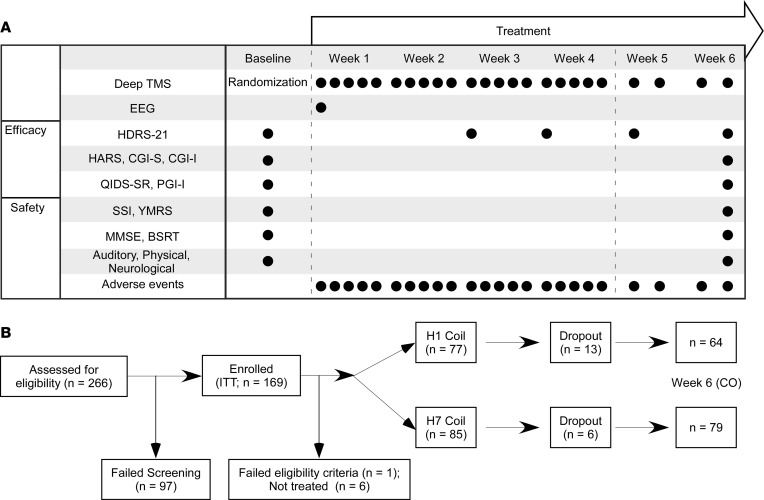
Figure 1. Timeline, assessments, enrollment, and randomization.
(A) Efficacy was assessed by Hamilton Depression Rating Scale (21-item version; HDRS-21), as well as by pre- and post-treatment Hamilton Anxiety Rating Scale (HARS) and Clinical Global Impression Severity and Improvement (CGI-S and CGI-I). In addition, patients completed self-report questionnaires on a weekly basis, including the Quick Inventory of Depressive Symptoms – Self Report (QIDS-SR) and the Patient Global Impressions Improvement (PGI-I). Daily functioning score (Global Assessment of Functioning, GAF) and quality-of-life measures (Quality of Life Enjoyment and Satisfaction Questionnaire, Q-Les-Q) were collected as well. Safety was assessed at pre- and posttreatment using the Scale for Suicide Ideations (SSI), Young Manic Rating Scale (YMRS), auditory threshold tests, physical and neurological examinations, and vital signs. Additional safety assessments included cognitive changes evaluations performed throughout the study, including the Mini-Mental Status Examination (MMSE) and the Buschke Selective Reminding Test (BSRT). Throughout the entire study patients were under the direct monitoring of a physician, and any adverse effects or subjective complaints were immediately recorded and treated. (B) Patients were outpatients aged 22 to 68 years who signed an informed consent form and had HDRS-21 ≥ 20 that was stable between screening and baseline assessments (±30%). Main exclusion criteria included comorbid psychiatric and neurological disorders, presence of psychosis, primary anxiety disorder causing higher distress than MDD, substance abuse within 6 months, prominent personality disorder causing higher distress than MDD, dysthymia, prior head trauma or seizures, and suicide attempt within 3 years. The intent-to-treat (ITT) analysis set included all patients enrolled in the study, and the completers (CO) analysis set included all patients randomized to the study who had no major protocol violations and completed the 6 weeks of treatment.