Fig. 4. UDCA is associated with lower levels of ACE2 and a better clinical outcome in patients with COVID-19.
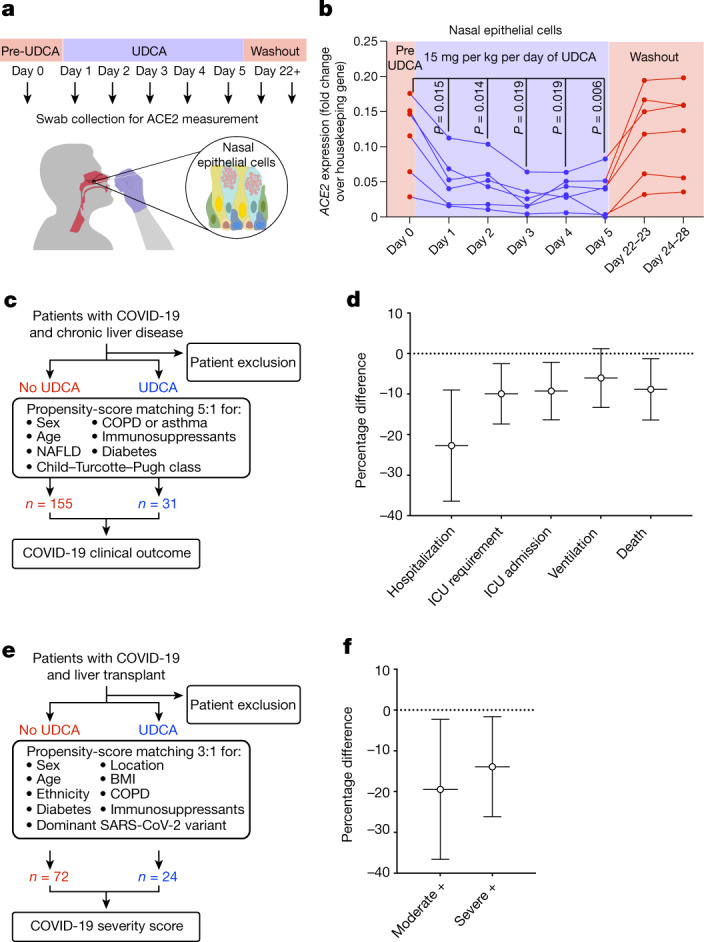
a,b, Schematic representation of the study design. Six healthy individuals received 15 mg per kg per day of UDCA for 5 days. ACE2 levels were measured by qPCR in nasal epithelial cells collected with nasopharyngeal swabs. Day 0 corresponds to samples collected immediately before starting UDCA treatment. Samples were collected daily during drug administration and again at day 22–23 and 24–28 to assess the washout of UDCA. b, qPCR measurement of the levels of ACE2 in nasal epithelial cells collected with nasopharyngeal swabs. Each dot represents one individual measurement; lines connect dots from the same individual (n = 6). Housekeeping gene, GAPDH; n = 6 individuals; one-way ANOVA with Geisser–Greenhouse correction. See Supplementary Table 5 for participant characteristics. c, Schematic overview of the analysis performed in the exploratory cohort corresponding to d (see Methods). d, Propensity-score-matched analyses showing major outcomes after SARS-CoV-2 infection in patients taking UDCA compared to control individuals not taking UDCA. n = 155 patients not on UDCA; n = 31 patients on UDCA. See Supplementary Tables 6 and 7 for patient characteristics. Bars, 95% CI. ICU, intensive care unit. e, Schematic overview of the analysis performed in the validation cohort corresponding to f (see Methods). f, Propensity-score-matched analyses showing disease severity after SARS-CoV-2 infection in patients taking UDCA compared to control individuals not taking UDCA, using the NIH COVID-19 severity score. Moderate +, moderate, severe or critical disease; severe +, severe or critical disease. n = 72 patients not on UDCA; n = 24 patients on UDCA. See Supplementary Table 8 for patient characteristics. Bars, 95% CI.
