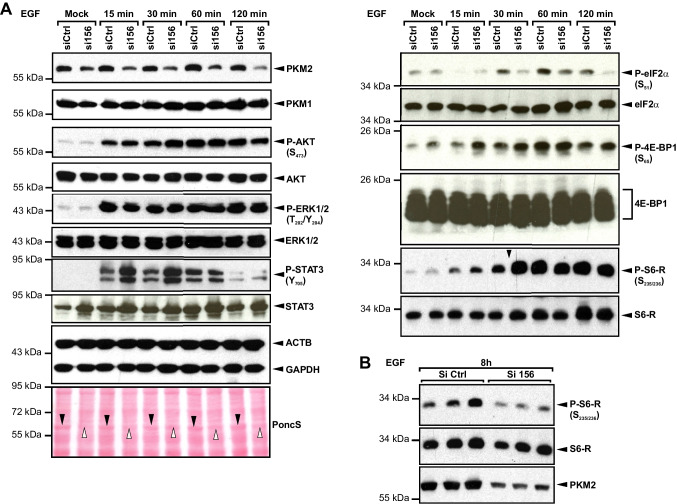
Fig. 7.
Silencing of PKM2 dysregulates growth factor activation of STAT3 and translational regulators. A HaCaT keratinocytes were transfected with PKM2-specific siRNA (si156) or control siRNA (siCtrl), and stimulated after 48 h by 30 ng/ml EGF for the indicated time points, and 25 µg of cell lysates were analyzed by immunoblot to assess the phosphorylation state of PKB (AKT) (Ser473), ERK1/2 (Thr202/Tyr204), STAT3 (Tyr705), eIF2α (Ser51), 4E-BP1 (Ser65), and S6 ribosomal protein (S6RP) (Ser235/236), and the expression level of the corresponding total protein. The silencing of PKM2 expression was verified by immunostaining of PKM2 and PKM1 proteins (top and second blots, respectively, left panel). The expression of ß-actin and GAPDH was used as a loading control. Ponceau S staining (PoncS, left panel, last blot) shows a decrease of a 60-kDa band (black downward arrowheads), subsequent to transfection with si156 (white upward arrowheads). A representative blot from three independent experiments is shown. B The phosphorylation state of S6RP (Ser235/236) at 8 h of stimulation by 30 ng/ml EGF was assessed in 25-µg cell lysate. Staining of total S6RP has been used as a loading control. PKM2 expression is shown in the lower blot. Results from three (n = 3) independent experiments are presented