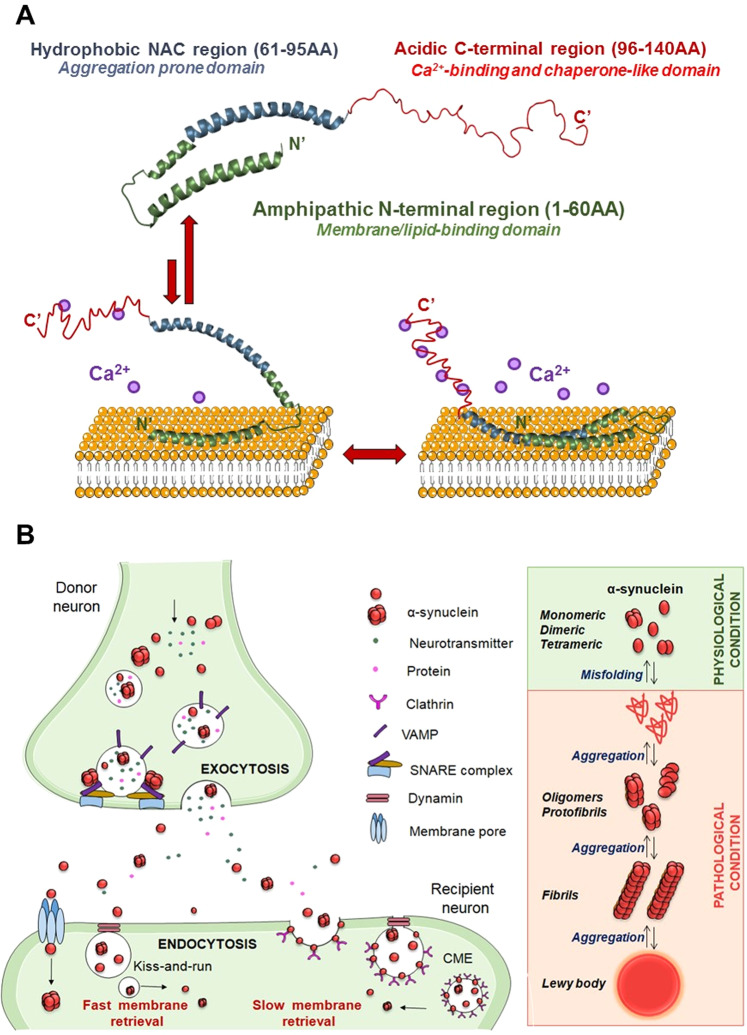
Fig. 1. Modular structure of α-syn and its dynamic interactions with membrane lipids in physiological and pathological conditions.
A Alpha-syn is structurally characterized by three modular regions: the N-terminal (green), characterized by amphipathic repetitions, is responsible for interactions with membranes; the hydrophobic NAC (blue), is relevant for aggregation, and the acidic C-terminal (red), is involved in Ca2+ binding and chaperon-like activity. In the presence of low concentrations of Ca2+, α-syn is stably anchored to the lipid surface via its N-terminal region only. With higher Ca2+ concentrations, also the NAC region, but not the C-terminus, shows lipid-binding properties, suggesting that it plays a different role in modulating the affinity of α-syn for cellular membranes. B Left panel: in control conditions, α-syn is important for regulating protein and neurotransmitter release by promoting SNARE complex formation and vesicle docking during the exocytosis process. Extracellular α-syn can affect recipient neurons by fast (i.e., kiss-and-run mode) and slow (i.e., clathrin-mediated endocytosis, CME) modes of membrane retrieval, as several endocytotic pathways can coexist in a single synapse. Interestingly, α-syn is associated with the CME of synaptic vesicles due to its role in sensing and stabilizing the curved membranes. Right panel: under physiological conditions (green), α-syn exists in different conformations balance between unstructured soluble monomeric and tetrameric forms. Under pathological conditions (light red), when the balance between α-syn generation and clearance is disrupted, α‐syn aggregates into oligomers, protofibrils, and fibrils, which further bring to the formation of protein inclusions called LB.