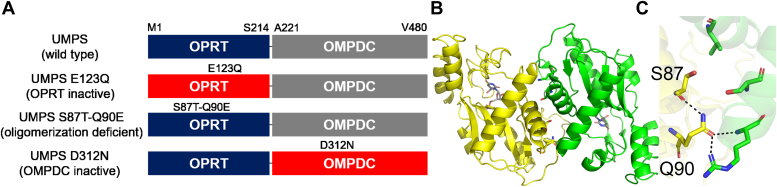
Figure 4.
UMPS domains and mutants.A, the two domains of UMPS are shown above with sequence numbers given to delineate the start and end points of the domains. The two native domains are shown in blue (OPRT) and gray (OMPDC), while domains with mutants are shown colored red. The specific mutations are shown above each domain at the approximate position in the sequence. Note that both the E123Q and D312N mutants are active site mutants of the OPRT and OMPDC domains, respectively. B, the structure of the OPRT dimer is shown (B) with the mutated residues shown as sticks (the OMP substrate is also given in grey to show the position of the active site). C, a zoomed in view of the location of the site (C) shows the mutated residues and the intraprotein and interprotein H-bonding interactions made. OMP, orotidine 5′-monophosphate; OMPDC, orotidine 5′-monophosphate decarboxylase; OPRT, orotate phosphoribosyltransferase; UMP, uridine 5′-monophosphate.