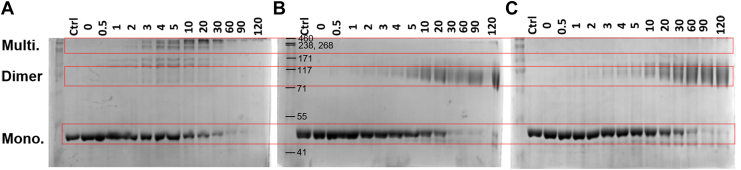
Figure 6.
Crosslinking of HsUMPS.A–C, native UMPS (A), the OPRT active site mutant (B), and the OPRT dimerization-deficient mutant (C) were treated with glutaraldehyde and analyzed by SDS-PAGE over time. For the native protein, several higher molecular weight multimers were visible within a few minutes after beginning the reaction. After 2 h, the protein was almost completely aggregated into large molecular weight forms. Conversely, for the two OPRT mutants, a species with a molecular weight consistent with a dimer was observed to be the predominant form, even after crosslinking for 2 h. The molecular weight markers are the same on all three gels and the corresponding weights are shown in B. The red boxes delineate likely monomers (Mono, expected MW 52 kDa), dimers (Dimer, expected MW 104 kDa), and multimers (Multi.). HsUMPS, human UMP synthase; OPRT, orotate phosphoribosyltransferase; UMP, uridine 5′-monophosphate.