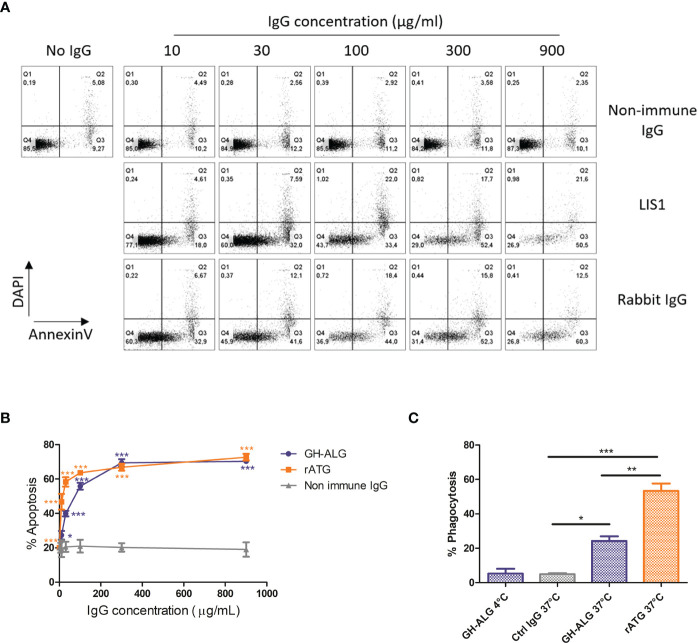
Figure 2.
Mechanisms of action of GH-ALG. Induction of apoptosis by GH-ALG. (A, B) Human PBMCs were treated with increasing doses (10, 30, 100, 300 and 900 µg/ml) of GH-ALG. Rabbit ATG and nonimmune DKO pig IgG were used as positive and negative controls, respectively. After 3 h of culture (37°C, 5% CO2), apoptosis was monitored by flow cytometry by Annexin V and DAPI staining. (B) The mean percentage of apoptosis was then determined by adding the percentage of cells in early (Annexin V+/DAPI-) and late (Annexin V+/DAPI+) apoptosis. The data are expressed as means ± SEM (N=3). Two-way ANOVA (*, p<0.5; **, p<0.01; ***, p<0.005), comparison of GH-ALG or rabbit ATG vs. nonimmune IgG. Phagocytosis assay. (C) Phagocytosis of opsonized PBMCs by human monocyte-derived macrophages was assessed by flow cytometry. CFSE-labeled cells were incubated with IgG in RPMI 10% FCS medium for 30 min at 4°C. Lymphocytes were washed twice and cultured with human macrophages (ratio 1:1) in RPMI 10% FCS. After 3 hours of culture, the cells were washed twice, and macrophages were labeled with CD14-BV421 for 30 min at 4°C. Cells were washed twice before flow cytometry analysis. Phagocytosis was assessed as the percentage of double‐positive (CFSE+/CD14+) cells. The values were compared by ANOVA followed by Tukey’s post hoc test. (*, p<0.5; **, p<0.01; ***, p<0.001).