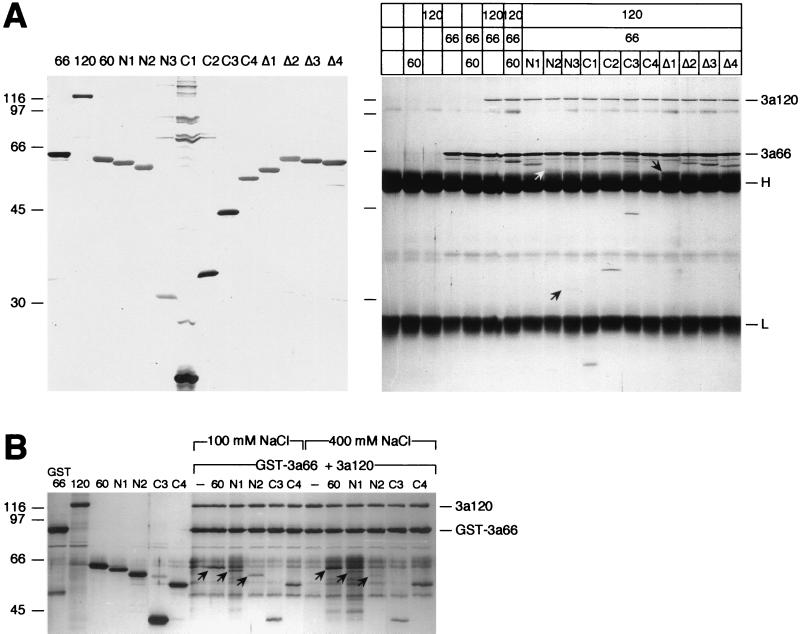
FIG. 3.
Amino acids 35 to 107 of SF3a60 are sufficient for its incorporation into SF3a. (A) Coimmunoprecipitation assay. Recombinant SF3a60 proteins were separated by SDS-PAGE and stained with silver (left panel). Identical amounts of the proteins were precipitated with mAb66 bound to protein A-Sepharose in the presence or absence of SF3a66 and/or SF3a120 as indicated above the figure. Bound proteins were separated by SDS-PAGE and visualized by silver staining (right panel). The positions of SF3a120, SF3a66, and the IgG heavy and light chains are indicated. Arrows point to 3a60-N2 and -N3, which bound inefficiently to SF3a120, and to 3a60Δ1 migrating just above the IgG heavy chain. The migration of molecular mass standards (in kilodaltons) is indicated to the left of each panel. (B) GST pull-down. Recombinant SF3a proteins (as indicated above the figure) were either loaded directly onto a SDS-polyacrylamide gel or incubated with GST-SF3a66 and SF3a120. Before elution of bound proteins, the gluthathione agarose was washed with buffer containing 100 or 400 mM NaCl as indicated. Arrows indicate full-length SF3a60, 3a60-N1, and 3a60-N2. The positions of SF3a120 and GST-SF3a66 are shown on the right, and the migration of molecular mass standards (in kilodaltons) is indicated on the left.