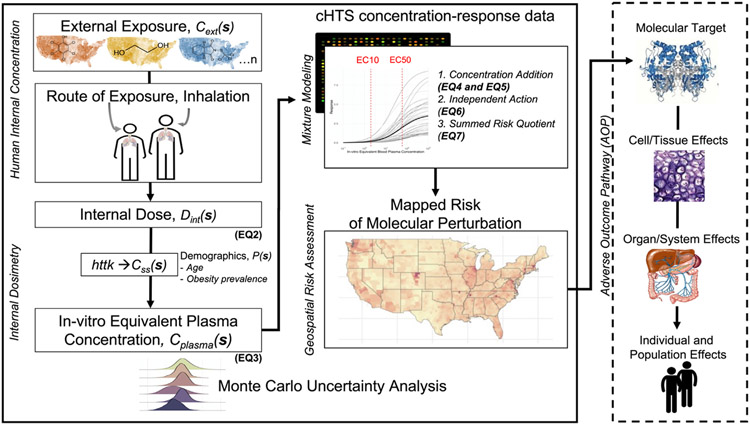
Fig. 1.
Schematic overview of the methods used to quantify the geospatial risk of molecular perturbation. The spatially explicit external chemical concentrations (Cext(s)) are converted to an internal dose (Dint(s)) via inhalation parameters and using county-specific distributions of age and obesity prevalence (P(s)). Dint(s) is then converted into an in vitro equivalent steady-state plasma concentration (Cplasma(s)) using a steady-state plasma conversion factor (Css(s)) estimated using physiological based toxicokinetic (PBTK) modeling. The dashed box indicates that P(s) influences both the inhalation rate and Css(s) used to calculate Cplasma(s). The geospatial risk assessment R(s) is developed by using the Cplasma(s) and concentration-response models generated using curated high-throughput (cHTS) data, which are integrated using mixtures models (e.g., concentration addition) to predict the risk of exposure. We use a probabilistic framework using a Monte Carlo uncertainty analysis. The mapped risk of molecular perturbation can be integrated with the Adverse Outcome Pathway (AOP) framework to link the molecular-level perturbation to an adverse outcome at the individual and population levels.