Abstract
In this issue of Cell, Ma et al. reveal a mechanistic role for PIEZO1 in iron homeostasis through molecular genetic mouse studies. They also demonstrate implications for human iron overload and deficiency syndromes, susceptibility to malarial infection, and red blood cell turnover in persons of African ancestries.
Iron is a key molecule in a number of critical cellular activities. Both deficiency and excess of iron can lead to pathological states with significant health consequences, underscoring the importance of iron homeostasis to basic cellular activities and human health. In this issue of Cell, Ma et al. (2021) undertake a series of gain-of-function (GOF) and loss-of-function (LOF) mouse experiments that provide unique insights to iron homeostasis. Combining this with clinical laboratory observations of iron metabolism in humans with a common GOF allele, they provide a fascinating tour of the emerging role of the gene PIEZO1 in iron homeostasis and potentially in human health.
PIEZO1 (OMIM #611184) encodes a protein of the same name that is a known cellular mechanotransducer—that is, it turns mechanical stimuli into downstream cellular activities (Kefauver et al., 2020). Specifically, PIEZO1 activation allows the non-selective movement of cations in the cardiovascular, hematopoetic, and osteoid systems. A rare GOF allele in PIEZO1 is known to result in iron overload in patients with hereditary xerocytosis (HX; MIM #194380), a rare red blood cell disorder. In considering the mechanisms underlying this observation, Ma and colleagues first recapitulated the iron overload phenomenon in mice through expression of a constitutionally active Piezo1. Interestingly, they found this to largely be a feature of aging mice. Given the central role of red blood cell and hemoglobin breakdown for iron metabolism, they focused their initial cellular explorations on the direct effects of PIEZO1 in red blood cells and were surprised to observe no iron-related defects in red blood cell-specific Peizo1 GOF mice.
Instead, their results pointed to a known but less heralded reservoir of PIEZO1 in macrophages (Winn et al., 2020). They found that macrophage-specific Piezo1 GOF leads to increased red cell turnover with concomitant increase of erythoferrone, decreased production of liver hepcidin, and a subsequent increase in serum and liver iron. This iron accumulation almost perfectly mimicked the extent and time of onset of constitutional Piezo1 GOF and was also seen in response to excessive iron exposure in young mice. Further, they showed that macrophage-specific Piezo1 GOF disrupts the normal rate of breakdown of red cells by macrophages—known as hemophagocytosis—by augmenting calcium influx and activating the pro-phagocytotic GTPase Rac1. They also found that they could rescue the GOF effect on hemophagocytosis using an inhibitor of PIEZO1 and that mice with macrophage-specific Piezo1 LOF had essentially the opposite hemophagocytic phenotype to GOF mice, thereby solidifying the crucial role of the molecule in iron homeostasis (Figure 1, left panel).
Figure 1. Putative roles of PIEZO1 in iron homeostatis and human health.
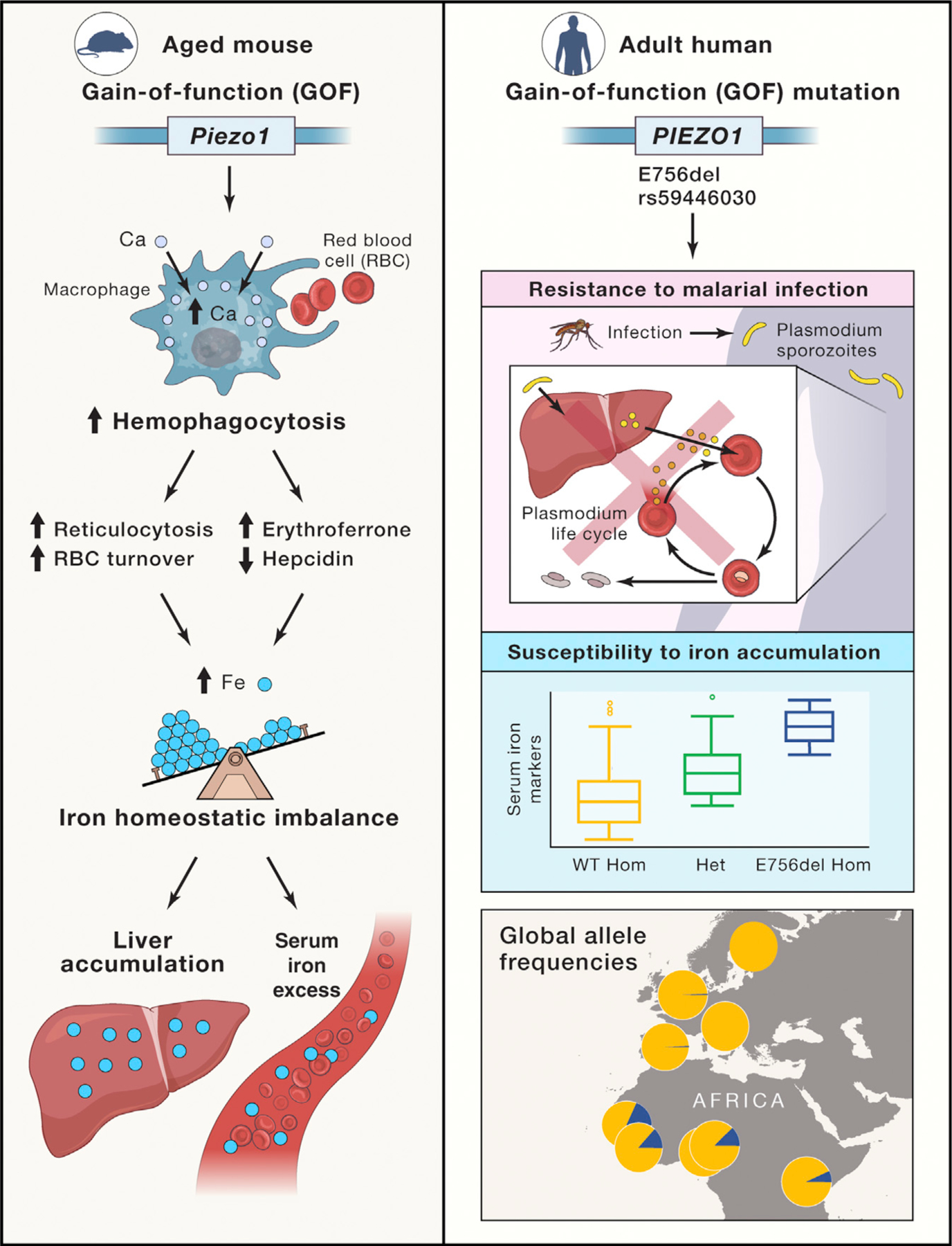
In mice (left panel), PIEZO1 activation leads to intracellular flux of calcium and hemophagocytosis of red blood cells. This in turn regulates levels of intermediates of red cell breakdown and iron uptake in the liver as part of iron homeostasis. Gain of function (GOF) of Piezo1 leads to increased serum iron and accumulation of liver iron stores. In humans (right panel), a PIEZO1 GOF allele (E756del) is associated with both resistance to malaria and increased serum iron markers and is common among populations of West African ancestry (https://popgen.uchicago.edu/ggv/). The yellow portion of each circle represents the frequency of the ancestral allele in the overlapped countries; the blue sector represents the derived E756del allele.
The authors then provided tantalizing clues of a similar mechanism in humans (Figure 1, right panel). They found that blood bank volunteers carrying a mild GOF PIEZO1 allele had increased levels of key iron markers, with some evidence of an allelic effect such that homozygous individuals tended to have the highest iron marker levels compared to individuals either heterozygous (next high) or homozygous (lowest) for the ancestral allele. Interestingly, this mild GOF allele—an in-frame 3-basepair deletion—is particularly common (frequency between 18% and 20% in available datasets; 1000 Genomes Project Consortium, 2015) among individuals of West African ancestries. Fascinatingly, the exact same allele was recently associated with protection from malaria in individuals from Gabon (Nguetse et al., 2020). Taken together, these observations suggest potential adaptive pleiotropy—where the effect of the allele differs depending on the environmental context; this could explain the fairly high frequency of the allele in individuals from West African countries where malaria is often endemic. Sickle cell disease is the classic example of this kind of pleiotropy; the HbS allele protects against severe malaria but causes a devastating hemoglobinopathy in the homozygous state. The same paradigm is also seen with allelotypes of APOL1, which protect from endemic trypanosomiasis or sleeping sicknesss but confer an increased risk for end-stage renal disease, particularly with HIV infection (Ekrikpo et al., 2020). Other examples of adaptive pleiotropy are emerging (Fan et al., 2016), particularly in the African context, and recent catalogs of selected genes and variants in Africa (Choudhury et al., 2020; Gurdasani et al., 2019) seem primed to produce similar stories in the future.
As with any exciting new study, the findings raise almost as many questions as they answer. It is unclear, for example, whether elevated iron in blood bank participants is clinically relevant; larger studies in diverse human cohorts will be needed to better define this link. Relatively little is known about iron accumulation in Africa—deficiency has tended to be the main focus of study—and the frequency of the variant across the continent is also unknown as a result of both the dearth of African genomic data and often extensive genetic variation by geography and ethnolinguistic group (e.g., among the Luhya in Kenya, the allele is seen half as frequently) (1000 Genomes Project Consortium, 2015). Similarly, precisely how PIEZO1 regulates hemophagocytosis or senses and transduces iron stress remains a mystery, although the interaction with calcium and in vitro pharmacological rescue provide valuable clues that might be therapeutically exploitable. The age-dependent phenomenon also raises intriguing translational questions: does it simply reflect cumulative, unchecked disruption of hemophagocytosis as seen in mice, or do environmental or dietary stressors also contribute? The mechanistic underpinnings of malaria resistance in PIEZO1 GOF carriers are also unknown. Is there, for instance, an interplay with any of the many hemoglobinopathy genes that also protect from malaria (e.g., alpha- or beta-thalassemia) and are similarly common?
The new findings suggest that assessing patients with clinically relevant iron accumulation of unknown or uncertain cause for other PIEZO1 GOF variants would be prudent, as would exploring cohorts of severe or refractory iron deficiency for putative LOF alleles. A central role for macrophages, hemophagocytosis, and mechanotranducers in iron homeostasis should also provide additional context for prioritizing candidates identified from genome-wide association studies of iron status (Benyamin et al., 2014; Li et al., 2015), thereby highlighting targets for future mechanistic studies.
The treatment of chronic iron overload remains challenging; patients require life-long phlebotomy, with only a few oral chelation drugs available. Iron deficiency and disorders of hemoglobin are among the most common causes of anemia in the world, and malaria remains a major cause of global childhood mortality. The reported findings sit squarely at the crux of these three classes of disorders, and thus have the potential to open new avenues of exploration across these critical areas of biology and medicine.
ACKNOWLEDGMENTS
Illustration developed in part with help from Adam Gillum, ARS, Children’s Nutrition Research Center, Baylor College of Medicine, Houston, TX. A.W. is funded by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH, USA), grant number 5U24HL135600-02. The views expressed are solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, and Abecasis GR (2015). A global reference for human genetic variation. Nature 526, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyamin B, Esko T, Ried JS, Radhakrishnan A, Vermeulen SH, Traglia M, Gögele M, Anderson D, Broer L, Podmore C, et al. ; InterAct Consortium (2014). Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat. Commun 5, 4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury A, Aron S, Botigué LR, Sengupta D, Botha G, Bensellak T, Wells G, Kumuthini J, Shriner D, Fakim YJ, et al. ; TrypanoGEN Research Group; H3Africa Consortium (2020). High-depth African genomes inform human migration and health. Nature 586, 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekrikpo UE, Mnika K, Effa EE, Ajayi SO, Okwuonu C, Waziri B, Bello A, Dandara C, Kengne AP, Wonkam A, and Okpechi I (2020). Association of Genetic Polymorphisms of TGF-b1, HMOX1, and APOL1 With CKD in Nigerian Patients With and Without HIV. Am. J. Kidney Dis 76, 100–108. [DOI] [PubMed] [Google Scholar]
- Fan S, Hansen ME, Lo Y, and Tishkoff SA (2016). Going global by adapting local: A review of recent human adaptation. Science 354, 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdasani D, Carstensen T, Fatumo S, Chen G, Franklin CS, Prado-Martinez J, Bouman H, Abascal F, Haber M, Tachmazidou I, et al. (2019). Uganda Genome Resource Enables Insights into Population History and Genomic Discovery in Africa. Cell 179, 984–1002.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefauver JM, Ward AB, and Patapoutian A (2020). Discoveries in structure and physiology of mechanically activated ion channels. Nature 587, 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lange LA, Duan Q, Lu Y, Singleton AB, Zonderman AB, Evans MK, Li Y, Taylor HA, Willis MS, et al. (2015). Genome-wide admixture and association study of serum iron, ferritin, transferrin saturation and total iron binding capacity in African Americans. Hum. Mol. Genet 24, 572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Dubin AE, Zhang Y, Mousavi SAR, Wang Y, Coombs A, Loud M, Andolfo I, and Patapoutian A (2021). A role of PIEZO1 in iron metabolism in mice and humans. Cell 184, this issue, 969–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguetse CN, Purington N, Ebel ER, Shakya B, Tetard M, Kremsner PG, Velavan TP, and Egan ES (2020). A common polymorphism in the mechanosensitive ion channel PIEZO1 is associated with protection from severe malaria in humans. Proc. Natl. Acad. Sci. USA 117, 9074–9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn NC, Volk KM, and Hasty AH (2020). Regulation of tissue iron homeostasis: the macrophage “ferrostat”. JCI Insight 5, e132964. [DOI] [PMC free article] [PubMed] [Google Scholar]


