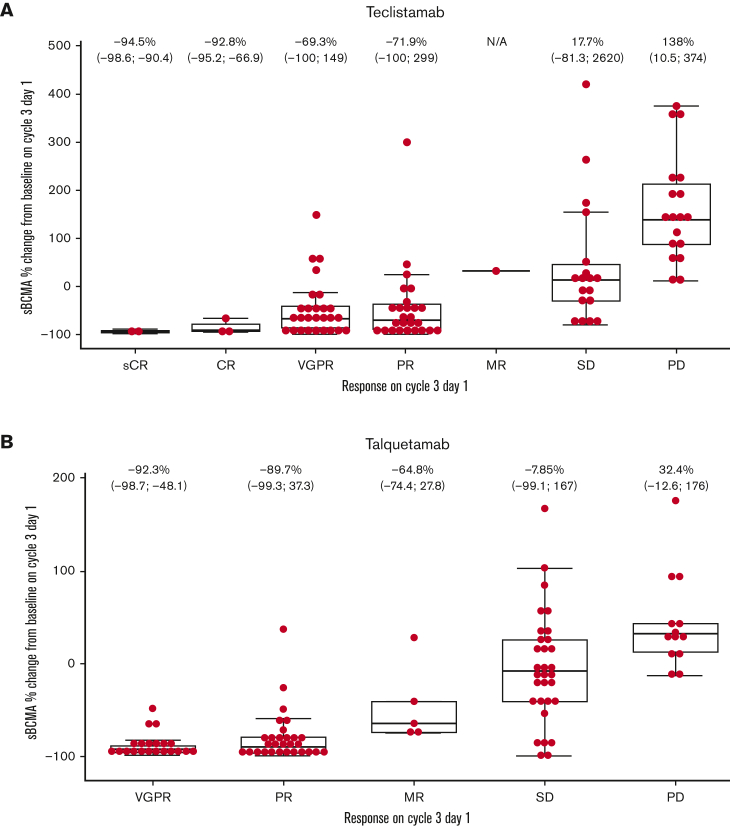
Figure 1.
Change in sBCMA levels on cycle 3, day 1 vs baseline according to depth of response to bispecific treatment. (A) sBCMA levels relative to depth of response for teclistamab. Patients received weekly intravenous (0.0003-0.72 mg/kg) or subcutaneous (80-3.0 mg/kg) doses of teclistamab. Cycle 3, day 8 data were used for 3 patients who had missing cycle 3, day 1 data. (B) sBCMA levels relative to depth of response for talquetamab. Cycle 3, day 8 data were used for 2 patients who had missing cycle 3, day 1 data. Data are not shown for 3 patients with sBCMA change >500% (508% [SD], 1201% [SD], and 2620% [SD]). Median percentage sBCMA change (minimum; maximum) shown. IV, intravenous; MR, minimal response; N/A, not applicable; PD, progressive disease; SC, subcutaneous; SD, stable disease.