Figure 3.
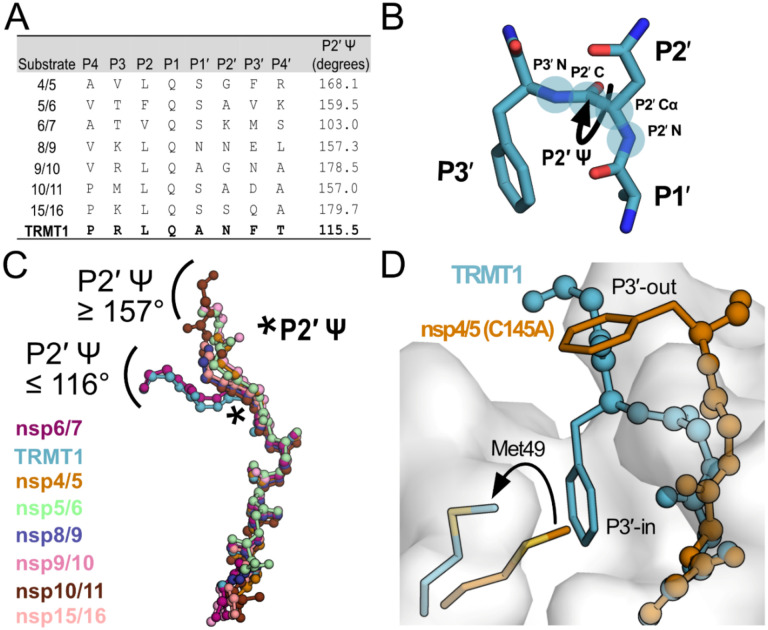
Analysis of Mpro-peptide structures illustrates two distinct substrate binding modes. A) Comparison of known Mpro substrate cleavage sequences and the P2′ Y backbone dihedral angles measured in the corresponding C145A Mpro-peptide structures for each substrate. We included all known C145A Mpro-viral peptide structures in this analysis, except those that were missing the P3′ residue or had poorly-defined electron density for the C-terminal portion of the peptide; structures used in this analysis are PDB IDs: 7MGS, 7T8M, 7DVW, 7T9Y, 7TA4, 7TA7, 7TC4, and 8D35. Additionally, since a C145A Mpro-nsp6/7 structure was not available, we used an H41A Mpro-nsp6/7 structure (PDB 7VDX) for this analysis. B) Section of an Mpro-bound peptide substrate showing residues P1′, P2′, and P3′, with the key P2′ Y dihedral angle illustrated with a curved arrow; the four backbone atoms that define the P2′ Y dihedral angle are labeled and highlighted with blue circles (P2′N–P2′Ca–P2′C–P3′N). C) Alignment of peptide substrate backbones in the Mpro active site reveals two distinct binding modes at the C-terminal end of the bound peptides characterized by P2′ Y dihedral angles 3 157° (nsp4/5, nsp5/6, nsp8/9, nsp9/10, nsp10/11, nsp15/16) or £ 116° (TRMT1, nsp6/7). Peptide overlays were generated by aligning SARS-CoV-2 Mpro-peptide substrate structures in PyMOL. The location of the P2′ Y dihedral angle in the substrate peptide backbone is denoted with a star. D) Alignment of nsp4/5- and TRMT1-bound Mpro structures showing divergent C-terminal peptide substrate binding modes in the Mpro active site. The backbone geometry of nsp4/5 (P2′ Y = 168°) positions the P3′ Phe sidechain away from the Mpro surface (‘P3′-out’ conformation), while the TRMT1 backbone geometry (P2′ Y = 115°) positions the P3′ Phe sidechain toward the Mpro active (‘P3′-in’ conformation) site where it displaces Mpro Met49 to open and occupy the S3′ pocket.