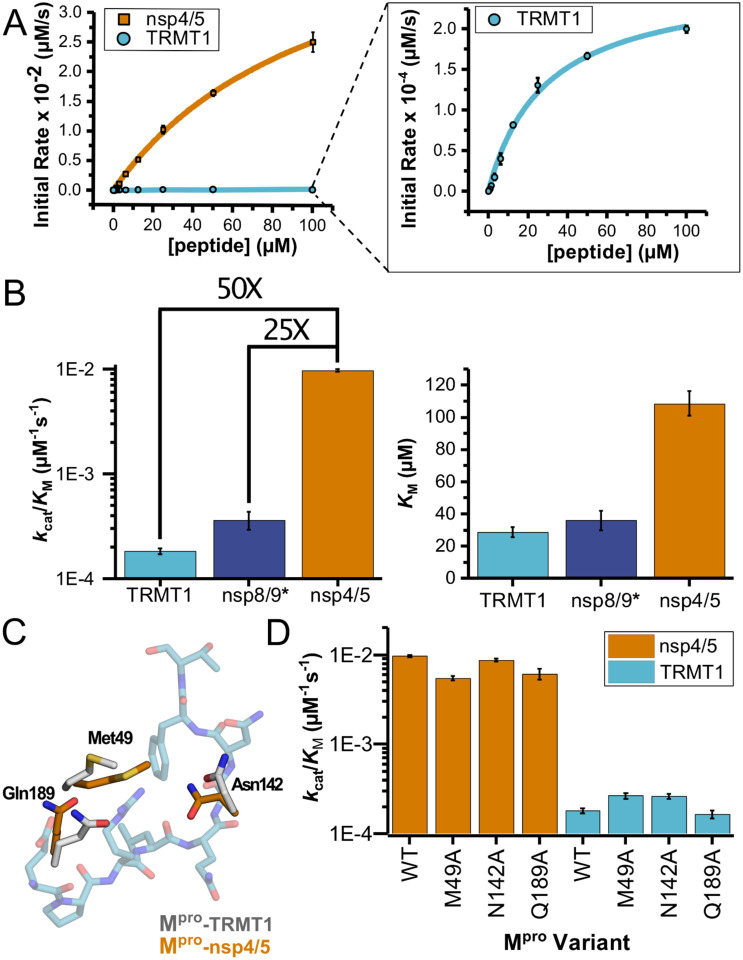
Figure 4.
Human TRMT1 peptides are cleaved with similar catalytic efficiencies to known Mpro substrates. A) Kinetics of nsp4/5 and TRMT1 peptide cleavage by Mpro. To initiate the reaction, 50nM enzyme was added to 100–0.097 μM peptide. Each fluorogenic peptide was conjugated with a quenching moiety, and upon peptide cleavage, the fluorescence of the cleavage product was measured to determine initial rates of the reaction. nsp4/5 rates were faster than those observed with the TRMT1 peptide. B) The catalytic efficiency of TRMT1 is similar to that reported of nsp8/9, though both of these substrates exhibit a large difference from nsp4/5. This suggests that TRMT1 is a feasible substrate of Mpro. *nsp8/9 kinetic data are from MacDonald et al. (15); these data were measured under similar assay conditions to our nsp4/5 and TRMT1 data and our nsp4/5 kinetic parameters agree closely with those measured by MacDonald et al.. C) Illustration of changes in Mpro Met49, Asn142, and Gln189 residue positioning in TRMT1-bound (white) versus nsp4/5-bound (orange) structures. The TRMT1 peptide is shown in blue; nsp4/5 peptide is not shown. D) No major changes in catalytic efficiency are observed for nsp4/5 and TRMT1 peptide cleavage upon mutagenesis of key Mpro residues involved in TRMT1 binding and recognition.