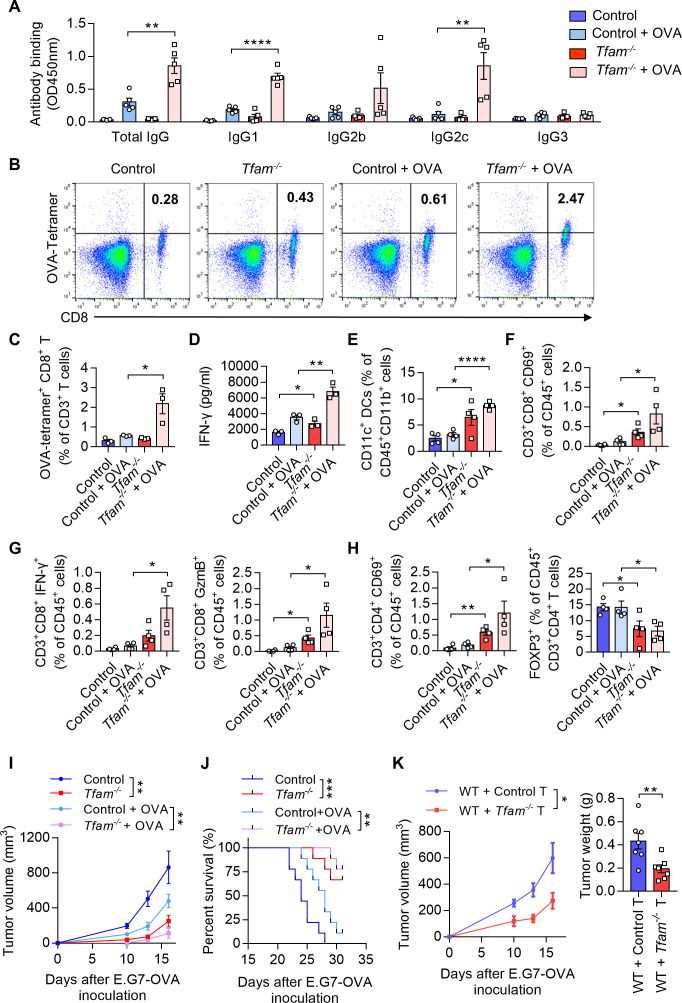
Figure 4.
TFAM deficient DC enhanced specific humoral and cellular immune responses. (A) Tfam deficiency enhances the specific anti-OVA humoral immunity. Control and Tfam-/- mice were vaccinated subcutaneously three times with or without 10 µg OVA antigen in PBS on days 0, 14, and 21. The mouse serum were collected on day 28 and levels of the total IgG and IgG subclasses were determined by ELISA. Serum antibody binding was determined by absorbance at 450 nm (n=5 mice). (B–D) Tfam deficiency enhances the specific anti-OVA cellular immunity. Splenic lymphocytes from immunized mice in (A) were isolated on day 28 and further incubated in vitro with CD8+ specific OVA257–264 peptides (10 µg/mL) for 72 hours. The generation of CD8+ CTLs was determined by FCM using PE-conjugated H-2Kb/OVA257–264 tetramer. Representative scatterplots of the OVA-specific CD8+ T cells gated from CD3+ cells are shown in (B) and quantified in (C). Level of IFN-γ in the supernatant was measured by ELISA (D) (n=3 mice). (E) Frequency of CD11c+ DCs in the spleen gated from CD45+ CD3- CD11b+ are determined (n=4 mice). (F) Frequency of CD3+ CD8+ CD69+ CTLs in the spleen gated from CD45+ are determined (n=4 mice). (G) Frequency of CD3+ CD8+ IFN-γ+ CTLs in the spleen gated from CD45+ are determined in the left panel, and frequency of CD3+ CD8+ GzmB+ CTLs in the spleen gated from CD45+ are determined in the right panel (n=4 mice). (H) Frequency of CD3+ CD4+ CD69+ T cells in the spleen gated from CD45+ are determined in the left panel, and frequency of FOXP3+ T cells in the spleen gated from CD45+ CD3+ CD4+ are determined in the right panel (n=4 mice). (I) Tfam deficient potentiates the antitumor effect of OVA vaccine in vivo. In the prophylactic model, control or Tfam-/- mice were immunized as in (A) (n=5 mice) and then injected subcutaneously with E.G7-OVA cells (5×105) 1 week after the third immunization. Tumor growth was monitored at the indicated times. (J) Tfam deficiency potentiates the survival of OVA-vaccinated tumor-bearing mice. Control and Tfam-/- mice were immunized with OVA and injected with E.G7-OVA (5×105) as previously described. Survival of mice was monitored daily (n=9 mice). (K) In the cellular adoptive therapy model, CD8+ T lymphocytes were isolated from immunized mice as in (A) on day 28 and subsequently injected intravenously into recipient mice, which were wild type mice subcutaneously inoculated with E.G7-OVA (5×105) cells. Tumor growth was monitored at the indicated times (left panel) and tumor weight was recorded after sacrifice on day 16 post-transplantation (right panel) (n=7 mice). Data represent the mean±SEM. Statistical significance was determined by two-way ANOVA in (I), left panel of (K) or a two-sided unpaired t-test in (A, C–H, right panel of K). Survival curve data in (J) were analyzed by log-rank (Mantel-Cox test). Representative results in (A–K) from two independent experiments are shown. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. ANOVA, analysis of variance; DC, dendritic cell; i.v., intravenous.