Abstract
BACKGROUND
Millions of human beings have suffered in the epidemic of Coronavirus disease 2019 (COVID-19), but until now the effective treatment methods have been limited.
AIM
This study aimed to evaluate the efficacy and safety of short-wave diathermy (SWD) treatment for moderate COVID-19 patients.
DESIGN
A prospective, double-blind, randomized controlled clinical study.
SETTING
Inpatients Unit of a COVID-19 specialized hospital.
POPULATION
Forty-two patients with moderate COVID-19 were randomly allocated at a 2:1 ratio to two groups: the SWD group and the control group.
METHODS
Participants of the SWD group received SWD treatment, and participants of the control group received placebo SWD treatment for one session per day, 10 minutes per session, for no more than 14 days. Both groups were given standard care treatment. Primary outcome was the rate of clinical improvement according to a seven-category ordinal scale. Secondary outcomes included the rate of computed tomography (CT) improvement and the rate of potential adverse events.
RESULTS
Clinical improvement occurred in 92.6% of patients in the SWD group by day 14 compared with 69.2% of patients in the control group (P=0.001). The Cox model indicated that the SWD group had a higher clinical improvement probability than the control group (hazard ratio: 3.045; 95% CI: 1.391-6.666; P=0.005). Similarly, CT improvement occurred in 85.2% of patients in the SWD group and 46.2% of patients in the control group respectively by day 14 (P=0.001). The Cox model indicated SWD group had a higher CT improvement probability than control group (hazard ratio: 3.720; 95% CI: 1.486-9.311; P=0.005). There was no significant difference in adverse events between the SWD group and the control group (2 of 27 [7.4%] SWD vs. 1 of 13 [7.7%] control, P=1.000), the most frequent of which were headache (1 of 27 [3.7%] SWD vs. 1 of 13 [7.7%] control patients) and dizziness (1 of 27 [3.7%] SWD vs. 0 of 13 [0%] control patients).
CONCLUSIONS
SWD is a valid and reliable adjuvant therapy with a favorable safety profile for moderate COVID-19 patients.
CLINICAL REHABILITATION IMPACT
Clinically relevant information is lacking regarding the efficacy and safety of SWD for patients with COVID-19. This study provides the first evidence that SWD is a promising adjuvant therapy for COVID-19.
Key words: Diathermy, Self efficacy, COVID-19
Attracting extensive attention since December 2019, a novel Coronavirus, designated SARS-CoV-2, has caused an international outbreak of respiratory illness termed Coronavirus disease 2019 (COVID-19). As of February 19, 2021, a total of 110.41 million people has been diagnosed globally, among which 2.44 million has died, indicating a mortality rate of about 2.21% (Coronavirus Resource Center of Johns Hopkins University). Even worse, scientists have recently tracked multiple mutations of SARS-CoV-2 that are potentially far more infectious. COVID-19 can affect a patient’s respiratory function, in severe cases resulting in the requirement of ventilator treatment or even death. Besides, about one third of the COVID-19 survivors showed a residual physical and functional impairment after hospitalization.1 However, current treatments, such as invasive mechanical ventilation,2 extracorporeal blood purification3 and antiviral therapy4 have shown limited effects. Although vaccination is underway across the world, new mutations of the virus may potentially not respond to the host of current vaccines. Based on clinical presentation, patients with COVID-19 in China are classified into four categories, including mild, moderate, severe and critical.5 Older patients and those with preexisting respiratory or cardiovascular conditions appear to be at increased risk of mortality from COVID-19.6
It has been reported that a more severe inflammatory cytokine storm resulted in greater severity of illness in patients with COVID-19.6 Short-wave diathermy (SWD) is a form of electrotherapeutic modality commonly used by physical therapists to suppress inflammation.7 A previous study has revealed that ultra-shortwave diathermy (USWD) can accelerate the repair of lung tissue and shorten the length of hospital stays for patients with severe acute respiratory syndrome.8 Thus, it was speculated that SWD might be effective for treatment of COVID-19. However, the use of SWD for COVID-19 is highly debatable because of the lack of research evidence to confirm its efficacy and rule out proposed side effects, such as pulmonary fibrosis.9 Consequently, in an effort to rigorously assess the efficacy and safety of SWD in the treatment of COVID-19, we performed a randomized controlled trial.
Materials and methods
Participants
Participants with moderate COVID-19 were recruited between March 1, 2020 and April 5, 2020 at the inpatient unit of Huoshenshan Hospital. The inclusion criteria were: 1) a positive reverse transcriptase–polymerase chain reaction (RT-PCR) assay for SARS-CoV-2 from a respiratory tract sample;10 2) moderate symptom category with fever, fatigue and respiratory symptoms;6 and 3) lung CT showing multiple patchy ground glass shadows or other typical manifestations.6 In consideration of the contraindications for SWD, patients with the following conditions were excluded: 1) need for Intensive Care Unit (ICU) monitoring or mechanical ventilation; 2) metal implants or tumor within the upper trunk; 3) abnormal skin sensation on the chest or upper back; 4) pregnant or lactating women; 5) severe cognitive impairment causing inability to complete treatment as directed or malignancy; and 6) lacking signed informed consent.
Ethical consideration
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committees of the Huoshenshan Hospital and the 940th Hospital of the Joint Logistic Support Force of the People’s Liberation Army (N. 2020KYLL088). All participants, or their legal representatives if they were unable to provide consent, provided written informed consent before participation.
Study design
As shown in Figure 1, this was a randomized, double blind, controlled trial. Using the computer-generated randomization method, eligible patients were assigned to the SWD group and control group at a 2:1 ratio. Since previous studies have suggested that SWD has a controlling effect on local inflammation,11 the 2:1 ratio was chosen to enhance enrollment and to obtain more safety information on the application of SWD. The patients did not know whether they were in the SWD group or control group until the study was completed. The physicians who assessed the patients during the study were blind to both groups. Only the SWD operator knew the participants allocated to which group because of the operative requirements of SWD.
Figure 1.
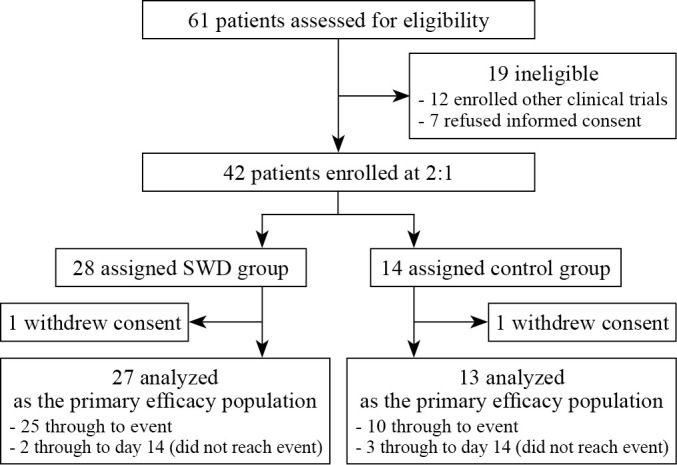
—Treatment study flowchart.
SWD utilized electromagnetic radiation at 27.12 MHz (XY-CDB-IV, Xiangyu Medical Equipment Co., Anyang, China). For the SWD group, the patients received a session of 10-min pulsed SWD treatment once a day for 14 days or less (in the case of discharge or death within 14 days). The pulse repetition rate was 350 Hz. The two short-wave electrodes were placed on the chest and upper back of the patient. For the control group, the patients received placebo SWD, electrodes positioned in the same manner as the SWD group but without turning on. The machine’s panel was directed out of the patients’ view. Standard care interventions, such us antivirals and antibiotic drugs, oxygen therapy, were provided to both groups if needed based upon the published guideline recommendations.12
Outcome measures
The primary outcome was the integrated clinical improvement rate. Clinical improvement (the event) was defined as a two-level decline on a seven-category ordinal scale within 14 days (or discharge or death, whichever came first) after randomization took place (day 0).13 The seven categories in the ordinal scale were: 1) not hospitalized with resumption of normal activities; 2) not hospitalized, but unable to resume normal activities; 3) non-ICU hospitalization, not requiring supplemental oxygen; 4) non-ICU hospitalization, requiring supplemental oxygen; 5) ICU hospitalization, not requiring extracorporeal membrane oxygenation (ECMO) and/or invasive mechanical ventilation; 6) ICU hospitalization, requiring ECMO and/or invasive mechanical ventilation; and 7) death.13 The clinical improvement rate was assessed once per day. Up to now, no randomized controlled trials have evaluated changes in the rate of clinical improvement according to the seven-category ordinal scale in patients with COVID-19. The sample size of our study was determined on the primary hypothesis of detecting an increase of 60% in clinical improvement rate of the SWD group compared with the control group by day 14. PASS software version 15 (NCSS LLC; East Kaysville, UT, USA) was used with a power of 0.8 and alpha of 0.05. Considering a dropout rate of 5%, the sample size is estimated to be 28 cases in SWD group and 13 cases in control group.
The secondary outcome was the integrated CT improvement rate. CT scans were performed on baseline (day 0), and again on day 14, discharge or death, whichever came first. There were three classifications of the CT images: 1) CT improvement was defined as the absorption of exudates, dissipating pulmonary consolidations and shrinking area of lesions; 2) CT progression was defined as more distributed consolidations, enlargement and fusion of lesions, and the observation of soft tissues opacities; and 3) CT stable was defined as no changes of density, lesion area and pulmonary consolidations.14 The classifications of the images were determined by a consensus between two researchers unaware of the grouping and treatment. According to a previous study,15 chest pain, cardiac arrhythmia, burns, and pulmonary fibrosis were chosen as potential adverse events, which were carefully observed during the study.
Statistical analysis
Continuous variables were expressed as medians and interquartile ranges (IQR) and were compared using the Mann-Whitney U Test. Categorical variables were expressed as number (%) and compared with a Chi-square test or Fisher’s Exact test. The distributions of each category of seven scales from admission to 14 days were shown in a grouped bar chart. The curves of clinical and CT improvement rates by time were portrayed by the Kaplan-Meier method. The Log-rank test and Cox proportional hazards regression model were utilized to compare the differences of the clinical and CT improvement rates between the two groups. Statistical analyses were performed using SPSS (version 22, IBM; Armonk, NY, USA) and R (version 4.0.3; R Foundation for Statistical Computing; Vienna, Austria). All statistical tests were two-sided and P values less than 0.05 were considered statistically significant.
Results
Patient characteristics and baseline data
Sixty-one patients were assessed for eligibility. 19 patients were excluded from the study, including 12 of whom participated in other clinical trials and 7 of whom refused to sign a written informed consent. Of the 42 patients who underwent randomization, 28 patients were assigned to SWD group and 14 patients to control group. One patient in each group withdrew consent from the study prior to its conclusion. Thus, a total of 40 patients entered into the final analysis (Figure 1). The baseline characteristics of the enrolled patients are shown in Supplementary Digital Material 1: Supplementary Table I. The demographics, level of seven-category scale, laboratory examinations and comorbidities were indistinguishable between the two groups.
Primary outcome measure
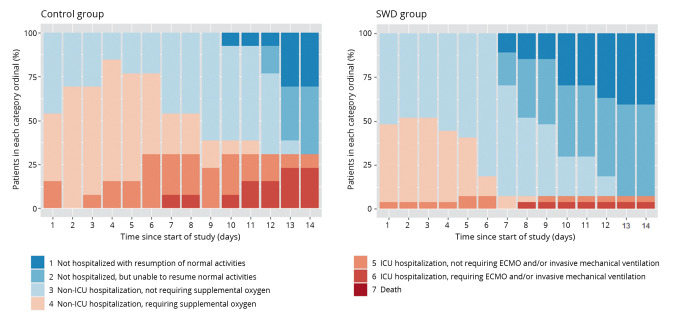
The distributions of the SWD group and the control group falling into each category of the seven-category scale throughout the study were shown in Figure 2. As time passed, a higher proportion of patients not hospitalized (categories 1-2) was observed in the SWD group than in the control group. Besides, the number of patients with severe outcomes (categories 5-6) in the control group increased gradually.
Figure 2.
—Distribution of patients in the seven-category scale from day 0 to day 14. SWD: short-wave diathermy; ICU: Intensive Care Unit; ECMO: extracorporeal membrane oxygenation.
The clinical improvement rate was 92.6% in the SWD group and 69.2% in the control group by day 14, and the difference was statistically significant (P=0.001). Additionally, patients in the SWD group had a significantly higher probability of clinical improvement than those in the control group (hazard ratio: 3.045; 95% CI: 1.391-6.666; P=0.005) (Figure 3).
Figure 3.
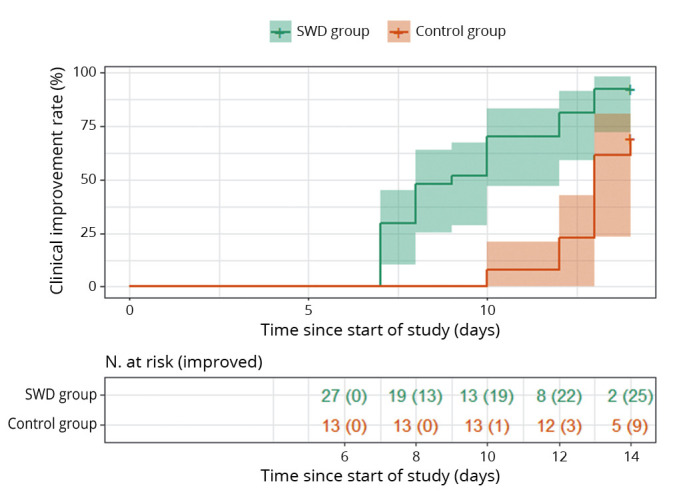
—Overall rate of clinical improvement among hospitalized patients in the SWD group and the control group.
Secondary outcome measure
Similarly, the CT improvement rate was 85.2% in the SWD group and 46.2% in the control group by day 14, and the difference was statistically significant (P=0.001). Patients in the SWD group had a significantly higher probability of CT improvement than those in the control group (hazard ratio: 3.720; 95% CI: 1.486-9.311; P=0.005) (Figure 4). Pulmonary infections in the SWD group were significantly reduced after treatment, with the significantly reduced scope and density of ground glass lesions reflected by the CT images (Figure 5). As shown in Table I, there was no significant difference in adverse events between the SWD group and the control group (2 of 27 [7.4%] SWD vs. 1 of 13 [7.7%] control patients, P=1.00). Pulmonary fibrosis, chest pain, cardiac arrhythmia and burns were not observed in either group during or after the SWD treatment. The most frequent symptoms were headache (1 of 27 [3.7%] SWD vs. 1 of 13 [7.7%] control patients) and dizziness (1 of 27 [3.7%] SWD vs. 0 of 13 [0%] control patients).
Figure 4.
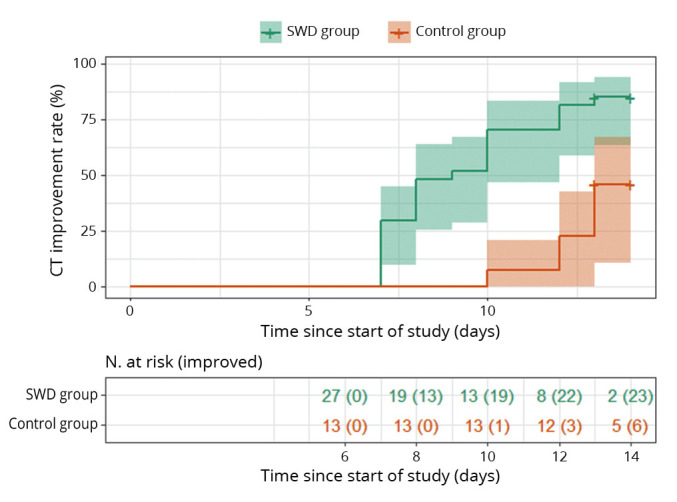
—Overall rate of CT improvement among hospitalized patients in the SWD group and the control group.
Figure 5.
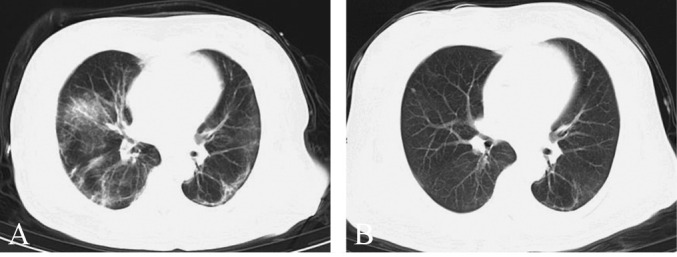
—Representative lung CT before and after SWD treatment. A) Before treatment; B) after treatment.
Table I. —Adverse events throughout the study in the two groups.
| Parameters | SWD group (N.=27) |
Control group (N.=13) |
P value |
|---|---|---|---|
| Overall, N. (%) | 2 (7.4) | 1 (7.7) | 1.000 |
| Pulmonary fibrosis | 0 | 0 | |
| Chest pain | 0 | 0 | |
| Cardiac arrhythmia | 0 | 0 | |
| Headache | 1 (3.7) | 1 (7.7) | |
| Dizziness | 1 (3.7) | 0 | |
| Burns | 0 | 0 |
Data are number of events (%). Patients with multiple events are counted once in each row.
Discussion
In our randomized controlled study, we confirmed for the first time that the addition of SWD significantly increased the probability of clinical improvement and CT improvement of the patients with moderate COVID-19 infection. Additionally, our safety data showed SWD was well-tolerated with no significant difference in adverse events compared with placebo SWD treatment.
SWD is a modality widely used in physical therapy for the management of inflammatory conditions, especially those caused by painful musculoskeletal disease. For example, adding SWD to a standard exercise regimen can reduce pain and improve function in patients suffering from chronic lateral epicondylitis.16 Our study revealed a significantly shorter turnaround time in both clinical manifestations and imaging findings of the COVID-19 patients in the SWD group compared with those in the control group, suggesting a good therapeutic effect of SWD for COVID-19. It is generally accepted that the high frequency waves of electromagnetic radiation provided by SWD can increase blood flow between the two electrodes, resulting in the anti-inflammatory and analgesic benefits.17 A single-blind, crossover trial demonstrated that pulsed short-wave therapy resulted in a significant increase in blood volume and skin temperature.18 Thus, the underlying mechanism of the COVID-19 improvement in the SWD group may be due to enhanced pulmonary blood flow and reduction of inflammation. Similar effects have been confirmed in other lung diseases, such as severe acute respiratory syndrome,8 bronchopneumonia,19 spontaneous pneumothorax,20 and chronic obstructive pulmonary disease.21, 22 Our study included the standard methods of care, such as the conventional drugs treatments, in the SWD group. Therefore, our results shed light upon the efficacy of the addition of SWD to the current standard care for the patients with COVID-19.
Besides efficacy, the excellent safety and tolerability profile of SWD should be noted. Current treatments for COVID-19 are mainly antiviral drugs, hormones and invasive mechanical ventilation.23 All of these treatments have certain limitations and side effects. Some medications deployed to treat COVID-19 (e.g., chloroquine, hydroxychloroquine, azithromycin, and ritonavir) can cause cardiac toxicity.24 Glucocorticoids may increase hyperglycemia and cause gastrointestinal bleeding and electrolyte disturbances.25 Endotracheal intubation is associated with an increased risk of infection while open systems or vented systems can increase the release of respirable particles.26 The benefit of physical therapy lays in its lack of drug-associated side effects as well its favorable therapeutic effect. However, the current passing through the thoracic cavity and the electrodes close to the skin may cause cardiac irregularity, chest pain, burn injury9 or pulmonary fibrosis.27 In our study, only minor side effects, including headache and dizziness, were observed in the SWD group, suggesting a favorable safety profile of SWD for COVID-19 patients.
In this study, we focused on patients with moderate COVID-19 infections for the following reasons: 1) improvement during the moderate stage of infection is conducive to preventing progression towards severe and critical infections; 2) severe and critical patients have serious symptoms, such as shortness of breath, respiratory failure and shock, which requires more interventions and leads to limitations for the application of SWD; 3) 71% of patients who died with severe COVID-19 presented complications associated with disseminated intravascular coagulation (DIC) and pulmonary congestion,28 making the risk-benefit ratio of SWD for these patients less clear; and 4) the symptoms of mild patients are not obvious, thus it is hard to distinguish therapeutic effects.
Limitations of the study
The study had several limitations. First, this study did not investigate the effects of different treatment parameters of SWD. Treatment times ranging from 10 to 30 min per day and machine parameters reported previously9 might result in different outcomes. Further study is required to determine the optimal parameters. Second, to enhance enrollment and obtain more safety information of SWD, the patients were randomly assigned to the SWD group and control group at a 2:1 ratio. However, placing more subjects in the SWD group relative to the control group reduces the statistical power, although the power dose not decline greatly since the ratio does not exceed 3:1.29 Third, it is known that some comorbidities such as chronic liver and kidney diseases are linked with an increased rate of mortality and severity in COVID-19 patients.30 Therefore, in our study, the rates of comorbidities were compared between the two groups and the result showed no significant difference. However, it should be noted that the control group had relatively more patients with chronic liver disease (1/13) and chronic kidney disease (1/13), compared with the SWD group (1/27 and 2/27, respectively). Although the prevalence of chronic liver and kidney disease is low and without significant difference between both groups, the potential influence of comorbidities to the outcome cannot be ruled out. Further studies with a larger sample size for the subgroup analysis of the comorbidities are needed.
Conclusions
Our results suggest that SWD induces significant clinical and CT improvements with few side effects for moderate COVID-19 patients, supporting SWD as a promising adjuvant therapeutic tool for managing COVID-19.
Supplementary Digital Material 1
Supplementary Table I
Baseline characteristics of patients
References
- 1.Baricich A, Borg MB, Cuneo D, Cadario E, Azzolina D, Balbo PE, et al. No-more Covid Group . Midterm functional sequelae and implications in rehabilitation after COVID-19: a cross-sectional study. Eur J Phys Rehabil Med 2021;57:199–207. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33565741&dopt=Abstract 10.23736/S1973-9087.21.06699-5 [DOI] [PubMed] [Google Scholar]
- 2.Hirayama A, Masui J, Murayama A, Fujita S, Okamoto J, Tanaka J, et al. The characteristics and clinical course of patients with COVID-19 who received invasive mechanical ventilation in Osaka, Japan. Int J Infect Dis 2021;102:282–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33127502&dopt=Abstract 10.1016/j.ijid.2020.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ugurov P, Popevski D, Gramosli T, Neziri D, Vuckova D, Gjorgon M, et al. Early Initiation of Extracorporeal Blood Purification Using the AN69ST (oXiris®) Hemofilter as a Treatment Modality for COVID-19 Patients: a Single-Centre Case Series. Rev Bras Cir Cardiovasc 2020. [Epub ahead of print] https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33113325&dopt=Abstract 10.21470/1678-9741-2020-0403 [DOI] [PMC free article] [PubMed]
- 4.Liu W, Zhou P, Chen K, Ye Z, Liu F, Li X, et al. Efficacy and safety of antiviral treatment for COVID-19 from evidence in studies of SARS-CoV-2 and other acute viral infections: a systematic review and meta-analysis. CMAJ 2020;192:E734–44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32493740&dopt=Abstract 10.1503/cmaj.200647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National-Health-Commission-National Medicine. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). Chinese Med J-Peking 2020;133:1087–95. 10.1097/CM9.0000000000000819 [DOI] [PMC free article] [PubMed]
- 6.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708–20. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32109013&dopt=Abstract 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jan MH, Chai HM, Wang CL, Lin YF, Tsai LY. Effects of repetitive shortwave diathermy for reducing synovitis in patients with knee osteoarthritis: an ultrasonographic study. Phys Ther 2006;86:236–44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16445337&dopt=Abstract 10.1093/ptj/86.2.236 [DOI] [PubMed] [Google Scholar]
- 8.Zhang LF, Zheng GX, Liu GL. Application of ultrashort wave diathermy in treatment of severe acute respiratory syndrome. Chin J Phys Med Rehabil 2003;25:14–6. [Google Scholar]
- 9.Yu HP, Jones AY, Dean E, Liisa Laakso E. Ultra-shortwave diathermy - a new purported treatment for management of patients with COVID-19. Physiother Theory Pract 2020;36:559–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32406778&dopt=Abstract 10.1080/09593985.2020.1757264 [DOI] [PubMed] [Google Scholar]
- 10.Poston JT, Patel BK, Davis AM. Management of Critically Ill Adults With COVID-19. JAMA 2020;323:1839–41. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32215647&dopt=Abstract 10.1001/jama.2020.4914 [DOI] [PubMed] [Google Scholar]
- 11.Laufer Y, Dar G. Effectiveness of thermal and athermal short-wave diathermy for the management of knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage 2012;20:957–66. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22659070&dopt=Abstract 10.1016/j.joca.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 12.Nicola M, O’Neill N, Sohrabi C, Khan M, Agha M, Agha R. Evidence based management guideline for the COVID-19 pandemic - Review article. Int J Surg 2020;77:206–16. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32289472&dopt=Abstract 10.1016/j.ijsu.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Fan G, Horby P, Hayden F, Li Q, Wu Q, et al.; CAP-China Network. Comparative Outcomes of Adults Hospitalized With Seasonal Influenza A or B Virus Infection: Application of the 7-Category Ordinal Scale. Open Forum Infect Dis 2019;6:ofz053. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30895200&dopt=Abstract 10.1093/ofid/ofz053 [DOI] [PMC free article] [PubMed]
- 14.Zheng Q, Lu Y, Lure F, Jaeger S, Lu P. Clinical and radiological features of novel coronavirus pneumonia. J XRay Sci Technol 2020;28:391–404. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32538893&dopt=Abstract 10.3233/XST-200687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu C, Peng RY. Biological effects and mechanisms of shortwave radiation: a review. Mil Med Res 2017;4:24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28729909&dopt=Abstract 10.1186/s40779-017-0133-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babaei-Ghazani A, Shahrami B, Fallah E, Ahadi T, Forough B, Ebadi S. Continuous shortwave diathermy with exercise reduces pain and improves function in Lateral Epicondylitis more than sham diathermy: A randomized controlled trial. J Bodyw Mov Ther 2020;24:69–76. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31987565&dopt=Abstract 10.1016/j.jbmt.2019.05.025 [DOI] [PubMed] [Google Scholar]
- 17.Goats GC. Continuous short-wave (radio-frequency) diathermy. Br J Sports Med 1989;23:123–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2691003&dopt=Abstract 10.1136/bjsm.23.2.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Mandeel MM, Watson T. The thermal and nonthermal effects of high and low doses of pulsed short wave therapy (PSWT). Physiother Res Int 2010;15:199–211. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20186887&dopt=Abstract 10.1002/pri.460 [DOI] [PubMed] [Google Scholar]
- 19.He YG, Ruan Q, Chang XM, Zhu Y. Changes of serum cytokines in children with bronchopneumonia treated with ultrashort wave diathermy. Zhonghua Shiyong Erke Linchuang Zazhi 2006;21:220–1. [Google Scholar]
- 20.Ma Y, Li J, Liu Y. Short wave diathermy for small spontaneous pneumothorax. Thorax 1997;52:561–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9227725&dopt=Abstract 10.1136/thx.52.6.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Sun QS, Xu SH, Liu XZ. Clinical study of ultrashort-wave therapy on airway inflammation in patients with chronic obstructive pulmonary disease. Chin J Phys Med Rehabil 2003;25:477–9. [Google Scholar]
- 22.Zhang Y, Sun QS, Wang W, Xu SH, Wang H, Zhou Q. Effects of ultrashort wave therapy on the clinical efficacy of noninvasive ventilation in patients with type II respiratory failure caused by chronic obstructive pulmonary disease. Chin J Phys Med Rehabil 2007;29:838–40. [Google Scholar]
- 23.Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol 2020;146:137–146.e3. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32470486&dopt=Abstract 10.1016/j.jaci.2020.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Million M, Lagier JC, Gautret P, Colson P, Fournier PE, Amrane S, et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis 2020;35:101738. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32387409&dopt=Abstract 10.1016/j.tmaid.2020.101738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye Z, Wang Y, Colunga-Lozano LE, Prasad M, Tangamornsuksan W, Rochwerg B, et al. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ 2020;192:E756–67. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32409522&dopt=Abstract 10.1503/cmaj.200645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeifer M, Ewig S, Voshaar T, Randerath WJ, Bauer T, Geiseler J, et al. Position Paper for the State-of-the-Art Application of Respiratory Support in Patients with COVID-19. Respiration 2020;99:521–42. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32564028&dopt=Abstract 10.1159/000509104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill J, Lewis M, Mills P, Kielty C. Pulsed short-wave diathermy effects on human fibroblast proliferation. Arch Phys Med Rehabil 2002;83:832–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12048663&dopt=Abstract 10.1053/apmr.2002.32823 [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): A case series. J Thromb Haemost 2020;18:1752–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32267998&dopt=Abstract 10.1111/jth.14828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torgerson DJ, Torgerson CJ. Unequal Randomisation. In: Designing Randomised Trials in Health, Education and the Social Sciences. London: Palgrave Macmillan; 2008. p.108–13 [Google Scholar]
- 30.Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in Patients with Liver and Kidney Diseases: An Early Systematic Review and Meta-Analysis. Trop Med Infect Dis 2020;5:80. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32429038&dopt=Abstract 10.3390/tropicalmed5020080 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table I
Baseline characteristics of patients