Figure 3.
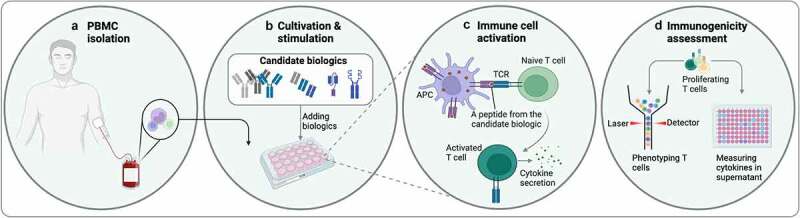
Schematic illustration of a Peripheral Blood Mononuclear Cell (PBMC) immunogenicity assay. A) PBMCs are isolated from healthy donors. B) Isolated cells are cultured in cell media with added candidate biologics. C) Candidate biologics are taken up by antigen presenting cells (APCs) and presented to T cells in culture. If the biologic is immunogenic, this may lead to T cell activation and concurrent cytokine secretion. D) Immunogenicity assessment can then be performed by phenotyping the T cells following stimulation with candidate biologics and measuring cytokine levels in culture supernatant.
