Figure 4.
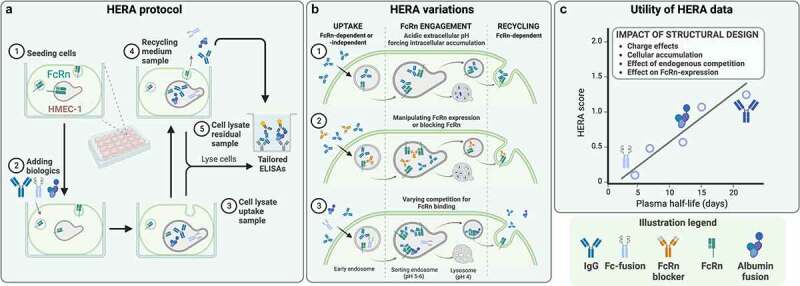
Human Endothelial Recycling Assay (HERA) as a tool for in vitro pharmacokinetic assessment and addressing FcRn-targeting strategies. A) Generalized HERA protocol. (1) Stably FcRn-transfected human microvascular endothelial cells (HMEC)-1 are seeded, prior to (2) adding FcRn-binding candidate biologics to two parallel cell plates. Following an incubation period, (3) cells from one plate are lysed to obtain an uptake sample. For the other plate, the media is exchanged to recycling medium, and after another incubation period, (4) the medium is harvested as a recycling sample, and (5) the cells are lysed to obtain a residual sample. Candidate biologics in all samples are quantified by an ELISA tailored for specific detection of the assessed biologic. B) Variations of the HERA protocol, enabling analysis of both FcRn-dependent and -independent uptake, cellular accumulation, and FcRn-dependent recycling. Variations include (1) performing the uptake step at mildly acidic extracellular pH, effectively forcing intracellular accumulation of biologics by preventing FcRn-mediated recycling, (2) manipulating FcRn-expression or blocking binding to FcRn to analyze FcRn-dependent and -independent cellular accumulation, and (3) introducing competition for FcRn binding to mirror endogenous competition on the ligand-binding sites of FcRn and its effects on the cellular transport for FcRn-binding biologics. C) HERA data can be used to address the impact of structural design of candidate biologics on FcRn-mediated cellular transport and unspecific cellular accumulation. For some candidate biologics, HERA data may allow for calculation of a score that correlates with plasma half-life in human FcRn-transgenic mice.
