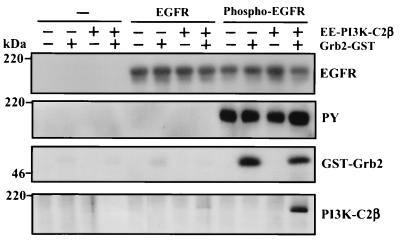
FIG. 10.
Formation of the EGFR-Grb2–PI3K-C2β complex in vitro. Recombinant EE-tagged PI3K-C2β (100 ng) was incubated in lysis buffer for 2 h at 4°C in the absence or presence of Grb2-GST fusion protein (100 ng) or GST. Immobilized EGFR, isolated by immunoprecipitation (Ab1; Oncogene Science) from lysates of confluent and quiescent cultures of A431 cells, was phosphorylated for 30 min at 30°C in protein kinase buffer upon addition of ATP. Either phosphorylated EGFR, nonphosphorylated EGFR, or mock immunoprecipitate was added to the Grb2–PI3K-C2β sample, and the incubation was continued for a further 1 h. Beads containing immobilized receptor and associated proteins were isolated by centrifugation, washed, fractionated by SDS-PAGE, and Western blotted with either anti-EGFR antibody, antiphosphotyrosine, anti-Grb2, or anti-PI3K-C2β antibody. PY, phosphotyrosine.