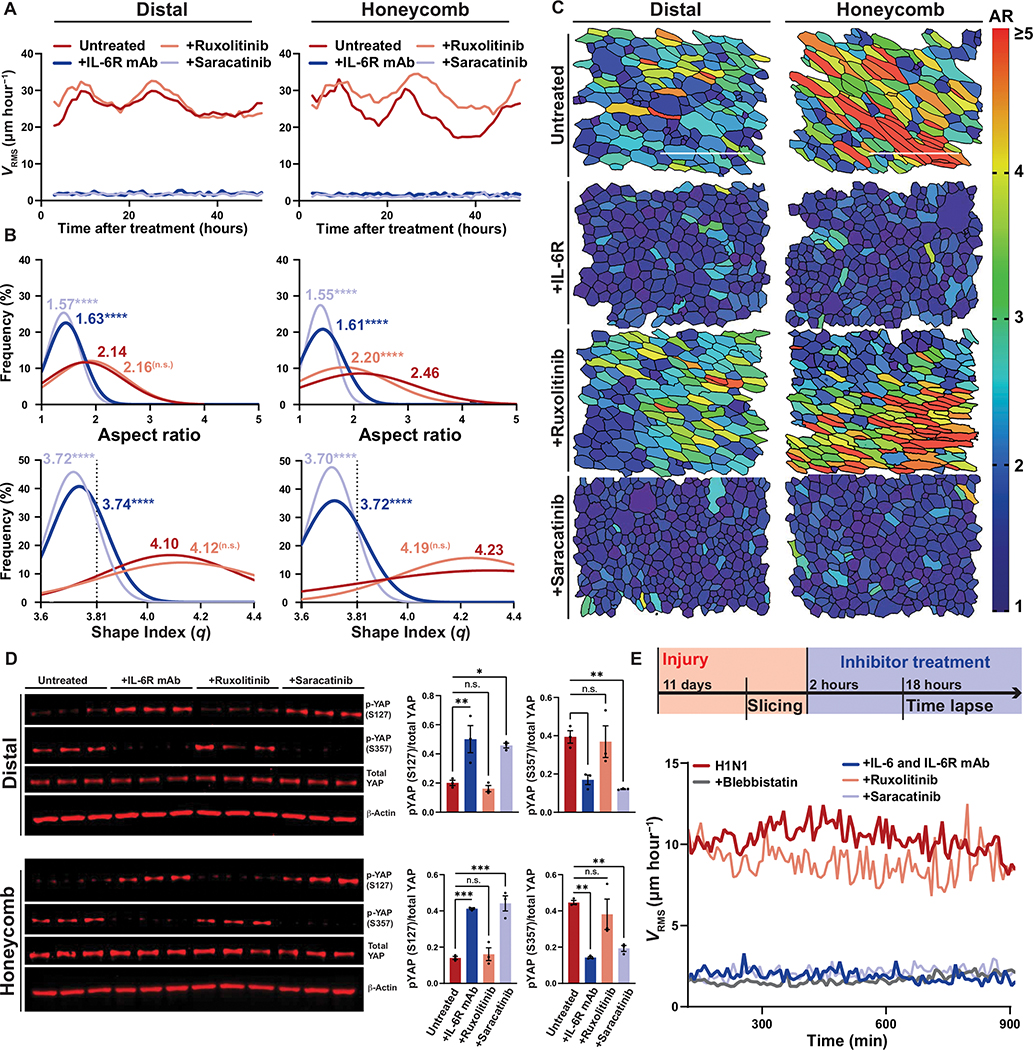
Fig. 4. Injured airways require IL-6/SFK for fluidization.
(A) Mean root-mean-squared velocity (VRMS) for distal and honeycomb cultures treated with anti–IL-6R antibody, ruxolitinib (JAK1/2 inhibitor), or saracatinib (SFK inhibitor). (B) Histogram of cellular aspect ratio (AR) and shape index (q) with mean values 48 hours after distal and honeycomb culture after inhibitor treatment. Dashed line represents shape index of 3.81, the theoretical threshold between fluid and solid phases. One-way ANOVA was used for statistical comparison. (C) Representative images of segmented distal and honeycomb cultures 48 hours after inhibitor treatment color coded on the basis of cell AR. Scale bars, 100 μm. (D) Western blot of inhibited distal and honeycomb cultures probed for YAP phospho-states after treatment with anti–IL-6R antibody, ruxolitinib (JAK1/2 inhibitor), or saracatinib (SFK inhibitor). Phospho-states shown signify YAP-cytoplasmic sequestration (S127) or SRC-dependent activation (S357). Error bars represent SEM. One-way ANOVA was used for statistical comparison. (E) Top: Timeline of Krt5-TdTomato mouse injury model and precision cut lung slicing (PCLS) with added inhibitor treatment. Bottom: Mean VRMS of PCLS treated with anti–IL-6/IL-6R antibodies, ruxolitinib, saracatinib, or blebbistatin. (A to E) For all statistical analysis n.s. (not significant), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Mouse experiments were independently performed N = 3 times with n ≥ 3 donors. Epithelial culture experiments were independently performed N ≥ 3 times with n ≥ 3 donors.