FIGURE 1.
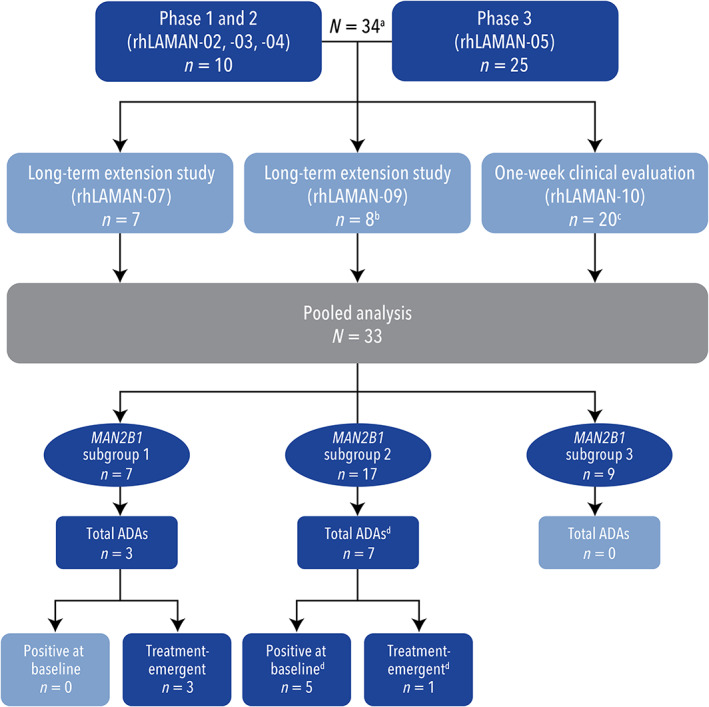
Patient‐flow diagram. (a) One patient participated in rhLAMAN‐02/‐03 and later participated in rhLAMAN‐05. This patient was only counted once. (b) One patient received four doses in rhLAMAN‐09 and then transferred to the compassionate‐use program and participated in the one‐week clinical evaluation. This patient was only counted once. (c) One patient participated in rhLAMAN‐05 in the placebo arm. They then entered the compassionate‐use program and discontinued shortly. As this patient had no data collected during the active treatment period, the patient was excluded from the integrated analysis. (d) One patient had ADA‐positive levels during placebo treatment but not at baseline or during treatment with VA.ADA, antidrug antibody; VA, velmanase alfa
