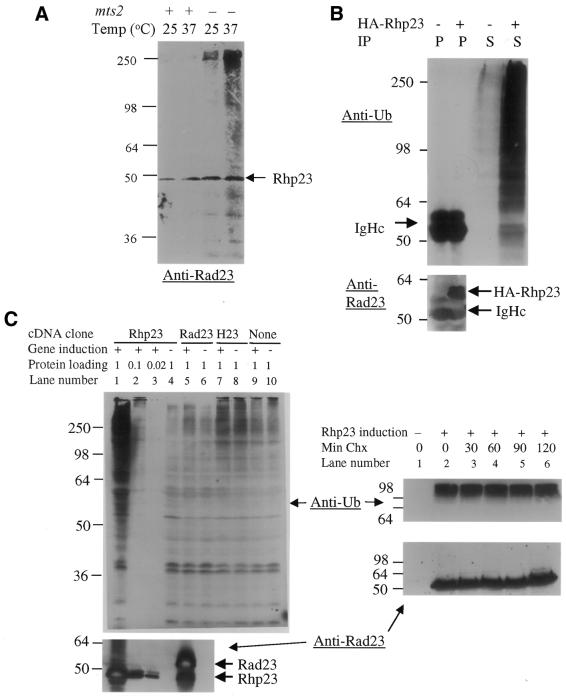
Figure 5.
Rhp23 is ubiquitinated at low levels, and overexpression of rhp23 leads to an accumulation of ubiquitinated proteins. (A) Ubiquitinated forms of Rhp23 are found only in a strain with a mts2 mutation in the proteasome subunit. The molecular weights of size standards (kDa) are indicated to the left of the immunoblot. (B) Overexpression of HA–Rhp23 leads to accumulation of large amounts of ubiquitinated proteins. Cell extracts were prepared from the wild-type strain SP223 without (–) or with (+) a plasmid carrying HA-tagged Rhp23 (HA–Rhp23). Cells were grown in media without thiamine to overexpress the tagged rhp23. Extracts were immunoprecipitated with anti-HA antibodies and the immunoprecipitate (indicated in the IP row by P) and the supernatant (indicated by S) were immunoblotted and probed with anti-ubiquitin (top panel) and anti-Rad23 antibodies (bottom panel). The positions of HA–Rhp23 and the immunoglobin heavy chain (IgHc), which was used as a protein loading control, are indicated. (C) In the left panel, cell extracts from the rhp23::ura4 strain carrying a plasmid with the rhp23, RAD23 or HHR23 cDNA or a vector control were prepared under inducing (+) or repressing (–) conditions for the nmt1 promoter expressing the cDNAs. For all extracts, 120 µg protein per lane were loaded on the gel, except for the induced rhp23 cDNA condition, where dilutions of 120, 12 and 2.4 µg were loaded (lanes 1–3). The upper immunoblot was probed with anti-ubiquitin antibodies. The lower immunoblot was probed with anti-Rad23 antibodies, which cross-react with Rhp23 but do not cross-react with HHR23A. The positions of Rhp23 and the slightly larger Rad23 are indicated. In the right panels, extracts were prepared from the rhp23::ura4 strain carrying a plasmid with the rhp23 cDNA either without (lane 1) or with induction (lanes 2–6). For the induced samples, cycloheximide (Chx) was added to 100 µg/ml at time 0 and samples taken 30, 60, 90 and 120 min later. Immunoblots with 120 µg protein were probed with anti-ubiquitin antibodies (upper panel) and with anti-Rad23 antibodies (lower panel). The samples in the right panel were run on a higher percentage polyacrylamide gel than in the left panel, so the anti-ubiquitin bands appear more compressed in the right panel.