FIGURE 2.
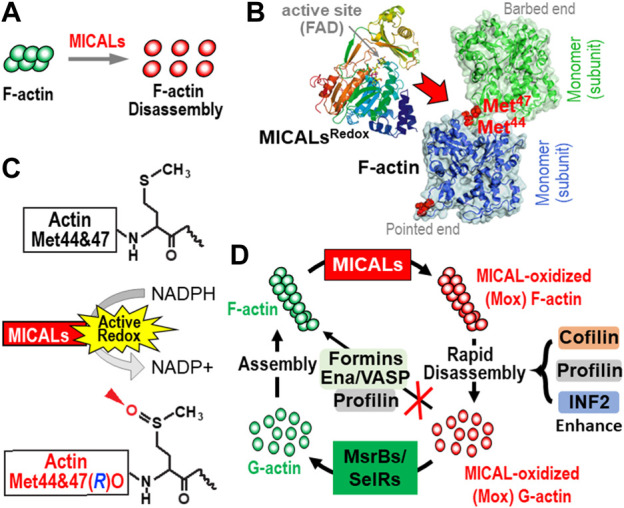
The MICAL’s activity and its effects on actin dynamics. (A–C) MICALs posttranslationally modify specific methionine (Met) residues in F-actin (red), which triggers F-actin disassembly (A). More specifically, MICALs bind to F-actin [(B), red arrow)] and in the presence of their coenzyme, NADPH (C), selectively and stereospecifically oxidize (O) actin’s Met44 and Met47 in the R-conformation [(C), arrowhead]. This oxidation of Met44 and Met47 [see (B), red] occurs in the D-loop, at the pointed end of individual actin filament subunits, which disrupts the interprotomers interactions in F-actin and leads to their rapid disassembly. (D) MICAL-mediated F-actin disassembly is regulated by other proteins. From top and clockwise: following MICALs’ oxidation of F-actin to generate MICAL-oxidized (Mox) F-actin, MICAL-triggered F-actin disassembly is enhanced by other proteins, including cofilin, profilin, and INF2. The Mox-G-actin that is formed does not readily re-polymerize even in the presence of profilin, formins, and Ena/VASP. Yet, Mox-G-actin is reduced specifically by selective methionine sulfoxide reductases (MsrBs/SelRs), and this G-actin can then re-polymerize normally. In this way, MICALs and MsrBs/SelRs create a reversible system for Redox regulation of actin dynamics in cells.
