Abstract
Background & Aims
Deep crypt secretory (DCS) cells are a critical component of the colonic stem cell niche. However, the regulatory mechanisms controlling DCS cell numbers and function are not well understood. Sprouty2 is an inflammation-responsive regulator of intracellular signaling that influences colonic secretory cell numbers in colitis via an epithelial–stromal interleukin (IL)33/IL13 signaling loop. Here, we tested the hypothesis that IL13, induced by epithelial Sprouty2 down-regulation, promotes DCS cell differentiation and function.
Methods
Distal colons from mice with an intestinal epithelial-specific Sprouty2 deletion (Spry2ΔIE) and littermate controls were analyzed by in situ hybridization for Reg4+ DCS cells. Single-cell RNA sequencing and immunostaining were used to identify DCS cell–derived host defense peptides (HDPs) and localization of IL13 and IL13 receptor; bulk RNA sequencing and quantitative polymerase chain reaction were used to quantify changes in expression of identified HDPs. Cytokine-treated colonoids were assessed for DCS cells. A requirement for an IL33/IL13 signaling loop in the regulation of DCS cells was assessed in vivo using IL13 null mice.
Results
Reg4+ DCS cell numbers were increased 2-fold in distal colons of Spry2ΔIE mice with a concomitant overall increase in DCS cell marker expression (Reg4, Spink4, and Agr2). Single-cell transcriptomics showed the HDP Retnlb/Resistin Like Beta (RELMβ) is highly enriched in DCS cells. Retnlb/RELMβ expression was increased in Spry2ΔIE colons. IL13, but not IL33, induced Reg4 and Retnlb expression in colonic epithelial organoids, and IL33-mediated expansion of the DCS cell population in vivo was dependent on IL13, which was expressed predominantly by type II innate lymphoid cells in the colonic mucosa.
Conclusions
Sprouty2 limits colonic DCS cell differentiation through suppression of IL13 signaling. At homeostasis, DCS cells are marked by high levels of the HDP RELMβ. Loss of epithelial Sprouty2 activates type II innate lymphoid cells to release IL13, promoting expansion of the DCS cell population and increased colonic RELMβ levels.
Keywords: IL13, ILC2, Deep Crypt Secretory Cell, RELMβ
Abbreviations used in this paper: DCS, deep crypt secretory; HDP, host defense peptide; IL, interleukin; ILC2, type II innate lymphoid cell; RELMβ, Resistin Like Beta; scRNA-seq, single-cell RNA sequencing; Spry2FF, Sprouty2 floxxed littermate control; Spry2ΔIE, intestinal epithelial-specific deletion of Sprouty2
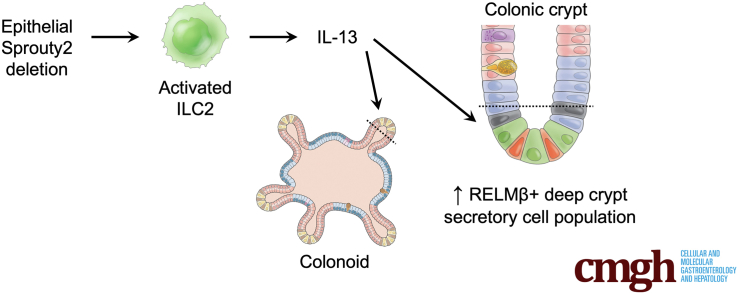
Graphical abstract
Summary.
Loss of epithelial Sprouty2 promotes interleukin 13–induced deep crypt secretory cell differentiation in the colon and expression of the host defense peptide, Resistin Like Beta (RELMβ), thus positioning this Sprouty2/interleukin 13 axis as an important regulator of secretory cell remodeling and function in the colon.
Colonic deep crypt secretory (DCS) cells are a specialized, organ-specific cell population located at the base of the colonic crypt. They are critical for the regenerative capacity of the colonic epithelium and share some similarities to Paneth and goblet cells in the small intestine.1 For example, DCS cells express mucin 2 (Muc2), similar to goblet cells, and are intercalated between crypt base cells, similar to Paneth cells. The receptor tyrosine kinase, c-Kit initially was proposed as a DCS cell marker,2 and subsequent work has shown additional markers including Reg4,1 although some ambiguity remains as to whether both of these markers label precisely the same cellular populations. The best-understood function of DCS cells at present is expressing Notch and EGF ligands that support the stem cell niche. However, because next-generation sequencing suggests these cells also express immunoregulatory genes (eg, Ccl6), it is likely they serve additional roles in maintaining colonic homeostasis.1,3 Whether DCS cells perform functions distinct from Paneth cells remains poorly understood. For instance, although DCS and Paneth cells overlap in expression of some gene products (Mmp7, Ccl6, Egf), unlike Paneth cells, DCS cells do not produce substantial Wnt ligand or contain remarkable lysozyme granules, suggesting they do not share the same profile of secreted factors. One understudied function of DCS cells is their potential production of host defense peptides (HDPs). This class of proteins is secreted from various cells of the body with a variety of roles including regulating immune cell function, the microbiota, and barrier function,4, 5, 6, 7 but it is unknown if DCS cells are an important source of these factors in the colon.
The signaling pathways controlling DCS lineage development are incompletely understood. Their development in the colonic epithelium can be driven by combined activation of Wnt and inhibition of Notch signaling in vitro.1 However, the environmental and immune signals controlling DCS cell abundance and function have yet to be identified. Because these cells likely play important roles in the maintenance of colonic epithelial health and the response to inflammation, defining the regulatory mechanisms controlling their numbers and function will be an important step toward elucidating their role in health and disease.
Sprouty2 is an inflammation-responsive regulator of growth factor signaling pathways that is highly expressed in the colonic epithelium.8 We previously showed that dynamic suppression of epithelial Sprouty2 by acute inflammatory signals initiates an epithelial–stromal interleukin (IL)33/IL13 signaling loop to drive rapid tuft and goblet cell expansion in the colon. This feedback mechanism positions Sprouty2 as an important regulator of secretory differentiation by controlling epithelial production of IL33. However, whether Sprouty2 and its subsequent control of IL33 and IL13 affects the abundance and function of DCS cells has yet to be tested.
Here, we used Sprouty2–null colonic epithelia as a tool to probe cytokine-mediated mechanisms controlling secretory cell differentiation. We performed unbiased analyses by bulk and single-cell RNA sequencing (scRNA-seq) to understand the impact of Sprouty2 on abundance and function of secretory lineages. We found that deletion of epithelial Sprouty2 leads to a substantial expansion of the DCS lineage and expression of the HDP RELMβ. Guided by these findings, we performed targeted mechanistic experiments to test the role of interleukin signaling in DCS differentiation and production of RELMβ. We report that IL13 induces expansion of the DCS cell population, thus positioning the Sprouty2/IL13 signal loop as a central mediator of secretory cell lineages in the colon. Furthermore, we show that RELMβ is a novel marker of DCS cells in the murine colon at homeostasis, suggesting a previously unappreciated role for DCS cells in producing this important HDP.
Results
Mice With an Intestinal Epithelial-Specific Deletion of Sprouty2 Have Increased Numbers of Reg4+ DCS Cells in the Distal Colon
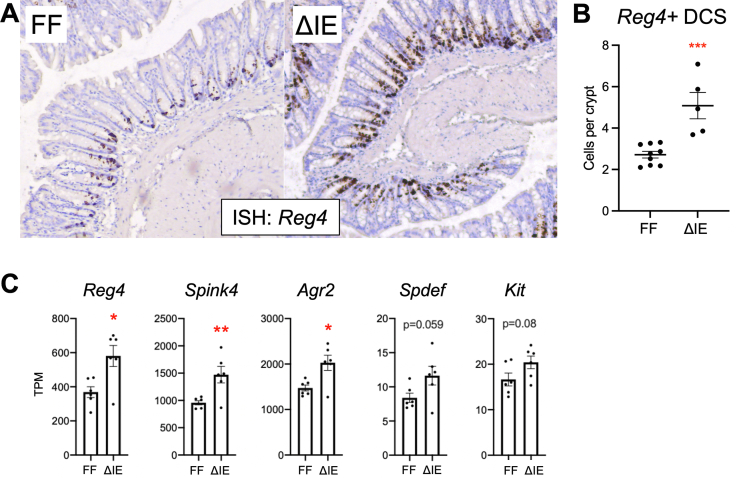
Secretory cells perform vital functions in maintaining the health and integrity of the colonic mucosa and the organism as a whole. We previously have shown that mice with an intestinal epithelial–specific deletion of Sprouty2 have expanded tuft and goblet cell lineages.8 DCS cells, located at the base of the crypt and marked by Reg4, are a recently described secretory lineage that express niche factors for colonic stem cells; ablation of Reg4+ cells leads to loss of colonic epithelial integrity.1 However, despite the apparent importance of this lineage, little is known about the signals that control their development or the functional mechanisms through which they support epithelial maintenance. To begin studying the role of Sprouty2 in these processes, we first performed in situ hybridization analysis for Reg4 (Figure 1A) on distal colons in mice with an epithelial-specific deletion of Sprouty2 (Spry2ΔIE) and littermate controls (Spry2FF). As expected, this staining confirmed localization of Reg4 to the base of the crypt. Quantification showed a doubling of Reg4+ DCS cells in Spry2ΔIE mice (Figure 1B). Analysis of other DCS markers in bulk RNA sequencing of colonic tissue from Spry2ΔIE and littermate controls8 showed significant increases in Reg4, Spink4, and Agr2 (Figure 1C), in line with increased numbers of these cells.
Figure 1.
Sprouty2ΔIEmice have increased numbers of Reg4+ DCS cells. (A) Distal colonic sections from Spry2ΔIE (ΔIE, n = 5) and control littermates (FF, n = 9) were analyzed by RNAScope in situ hybridization for the DCS cell marker Reg4, quantified in panel B. (C) Bulk RNA-seq on distal colonic tissue was analyzed for expression levels of DCS cell markers. n = 6 per genotype. Data were analyzed by 2-tailed Student t test. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. TPM, transcripts per million.
Retnlb Is Highly Expressed in DCS Cells, With Minimal Homeostatic Expression in Other Epithelial Cell Types
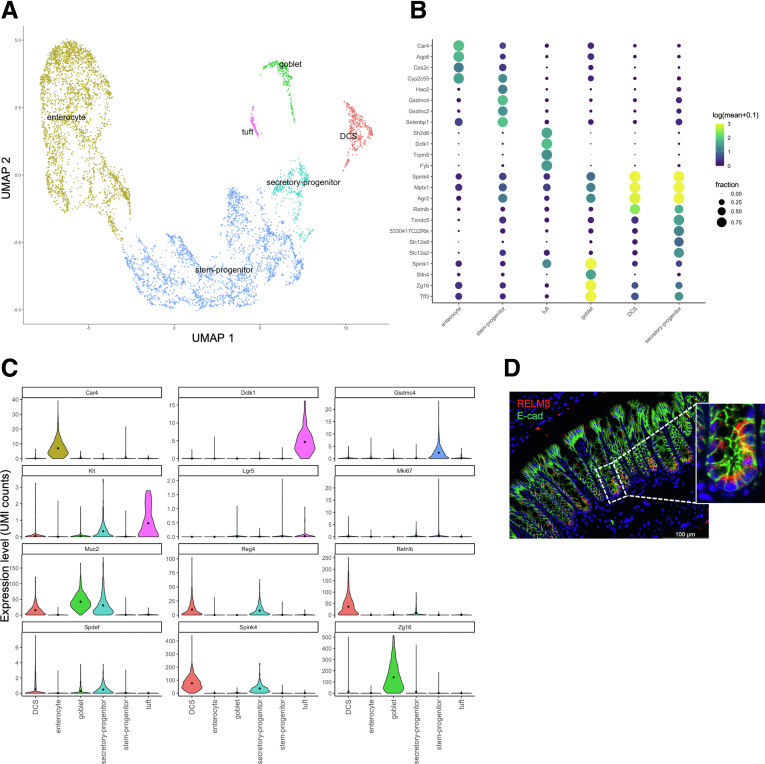
We next wanted to find clues as to what functional effects may result from changes in DCS cell number by analyzing gene expression in this population. We performed an unbiased scRNA-seq analysis of colonic tissue from Spry2FF control (functionally wild-type) mice. This allowed for characterization and determination of novel DCS-associated transcripts. As expected, this analysis identified specific clusters of stem and progenitor cells, secretory progenitors, tuft cells, goblet cells, DCS cells, and absorptive enterocytes within the epithelial population (Figure 2A). Gene expression analysis showed distinct patterns of high expressing transcripts within each of these clusters (Figure 2B).
Figure 2.
Single-cell transcriptomic analysis of colonic epithelial cells shows Retnlb/RELMβ marks DCS cells at homeostasis. Single-cell transcriptomics was performed on 26,848 isolated distal colonic single cells from Spry2FF control mice (n = 4 mice). (A) The total survey of cells was pooled, clustered, and filtered to retain colonic epithelial cell populations (6502 cells), which are shown after uniform manifold approximation and projection (UMAP). (B) Expression intensity dot map representing gene expression based on the fraction of cells that were positive and abundance of gene expression within each cluster. (C) Expression levels of gene markers for cells residing within each cluster are shown as violin plots. (D) Immunostaining for RELMβ in colonic crypts. E-cad, E-cadherin; UMI, unique molecular identifier.
Next, we further analyzed the DCS cell cluster to look for genes specifically enriched in these cells. By differential gene expression analysis between epithelial clusters, we found multiple gene products enriched in the DCS cell lineage, including previously identified markers Reg4 and Spink4 (Figure 2C). In contrast to these markers, Kit was expressed more broadly in the epithelium, suggesting it is not in fact a specific DCS cell marker. Notably, this analysis also showed that one of the highest differentially expressed genes in the DCS cell cluster was Retnlb, which encodes the HDP RELMβ (Figure 2C). To confirm localization of translated RELMβ protein, we performed immunofluorescence analysis on distal colonic sections. In contrast to previous studies showing RELMβ expression in goblet cells in colitis or parasitic infection,5,9 we found that under homeostatic conditions RELMβ localizes predominantly to cells at the colonic crypt base (Figure 2D), consistent with expression in DCS cells. Neither Retnlb transcript nor RELMβ protein was expressed robustly by goblet cells (Figure 2B–D).
RELMβ Expression Is Increased in the Colon of Spry2ΔIE Mice
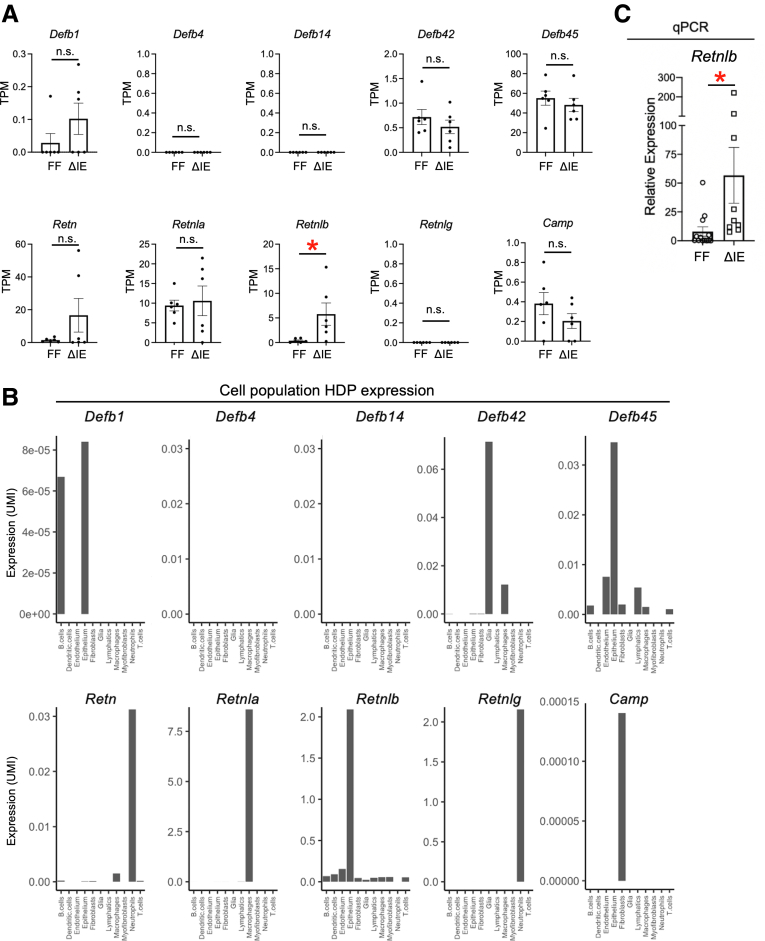
HDPs are a broad class of secreted proteins with known roles in microbe regulation, barrier function, and tissue immunity in the intestine and other tissues, and multiple families of HDPs are expressed across different tissues of the body.10 Because we were interested in further defining the role of DCS cells in HDP production, we performed an unbiased expression analysis of HDPs in the colon using our previously published colonic bulk RNA sequencing data set8 (GSE160854). This analysis found detectable expression of 7 HDPs: β-defensin 1 (Defb1), β-defensin 42 (Defb42), β-defensin 45 (Defb45), resistin (Retn), RELMɑ (Retnla), RELMβ (Retnlb), and Camp (cathelicidin) (Figure 3A) in Spry2ΔIE and littermate control mice. Of these, only Retnlb, Defb1, and Defb45 showed epithelial expression in our colonic scRNA-seq data set (Figure 3B). Furthermore, only Retnlb/RELMβ was increased significantly in Spry2ΔIE mice (Figure 3A). Because our scRNA-seq data show that Retnlb/RELMβ is highly expressed in DCS cells (Figure 2B and C) and is increased in Spry2ΔIE colons in our bulk RNA-seq data set (Figure 3A), we analyzed Retnlb expression levels in additional litters of Spry2ΔIE and littermate controls by quantitative polymerase chain reaction (Figure 3C). This confirmed that, in a larger cohort, Retnlb was induced significantly in the colons of Spry2ΔIE mice. Together, these data show that Retnlb is enriched specifically in the epithelium, highly concentrated in DCS cells at homeostasis, and selectively regulated by Sprouty2.
Figure 3.
Retnlb/RELMβ is expressed in DCS cells at homeostasis and is up-regulated by deletion of Sprouty2. HDP expression was assessed in our bulk RNA-seq data set generated from distal colons of Spry2ΔIE and control littermates (GSE160854). (A) Expression of multiple HDPs (Defb1, Defb42, Defb45, Restn, Retnla, Retnlb, and Camp) was detected in the colon. (B) Analysis of HDP expression in cellular populations identified in colonic scRNA-seq showing enrichment of Defb1, Defb45, and Retnlb in the epithelium. (C) Colonic samples from a larger cohort of Spry2ΔIE (n = 9) and control (n = 12) littermates were analyzed by quantitative polymerase chain reaction (qPCR) to test for RELMβ (Retnlb) expression levels. Data were analyzed by 2-tailed Student t test. ∗P < .05. TPM, transcripts per million; UMI, unique molecular identifier.
The Colonic Type II Innate Lymphoid Cell Population Is Activated in Spry2ΔIE Mice
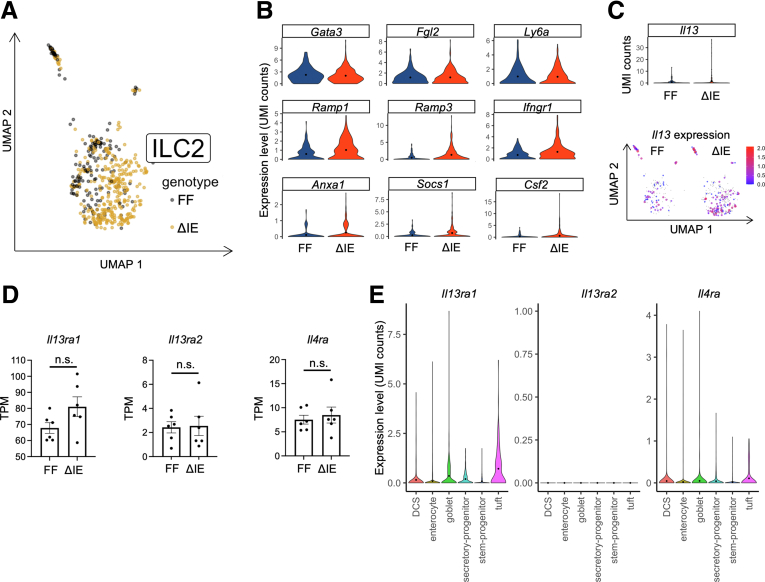
IL13 levels are increased in the colons of Spry2ΔIE mice.8 Type II innate lymphoid cells (ILC2s) are an IL13-expressing immune cell subtype with important roles in tissue homeostasis and remodeling. We previously showed that Spry2ΔIE colons have increased levels of IL13, have increased numbers of Il13-expressing and Gata3-expressing cells, and express an ILC2-enrichment signature in bulk RNA-seq analysis.8 However, whether this increase in the ILC2 population reflected a change in the activity of these cells remained unclear. Using our scRNA-seq data set, we asked whether the ILC2 population in Spry2ΔIE mice has an altered activation state by analyzing differential gene expression in these cells. The ILC2 cluster (characterized by high expression of Gata3, Lmo4, Ptpn13, and Il1rl1)11, 12, 13 showed a distinct separation between control and Spry2ΔIE mice by uniform manifold approximation and projection analysis (Figure 4A). Differential gene expression analysis showed that although baseline ILC2 identity markers (Gata3, Fgl2, and Ly6a)14 remain relatively unchanged, genes associated with altered ILC2 activation (Ramp1, Ramp3, Ifngr1, Anxa1, Socs1, and Csf2)14, 15, 16, 17, 18, 19 and expression of Il13 are increased in colonic ILC2s from Spry2ΔIE mice (Figure 4B and C).
Figure 4.
Sprouty2ΔIEmice have activated ILC2s in the colon. (A) Transcriptomic analysis of ILC2s obtained from single-cell sequencing of colonic mucosal isolate from Spry2ΔIE (n = 303 cells) and control (n = 136 cells) mice representing 1.12% and 0.51% of the total cells recovered for each genotype, respectively, and shown as uniform manifold approximation and projection (UMAP). (B) Violin plots showing expression of ILC2 identity genes (Gata3, Fgl2, and Ly6a) and ILC2 activation markers (Ramp1, Ramp3, Ifngr1, Anxa1, Socs1, and Csf2) in Spry2ΔIE and control mice. (C) Analysis of Il13 expression in the ILC2 populations of Spry2ΔIE and control mice by violin plot and UMAP. (D) Expression of IL13 receptors in bulk RNA-seq on distal colons on mice. (E) Cell-specific expression levels of the IL13 receptors, Il13ra1 and Il4ra, and inhibitory receptor, Il13ra2, were analyzed in the colonic scRNA-seq data set, showing broad expression of Il13ra1 and Il4ra across epithelial cell types, including DCS cells, and no expression of Il13ra2 in the epithelium. Data were analyzed by 2-tailed Student t test. TPM, transcripts per million; UMI, unique molecular identifier.
To confirm that epithelial lineage allocation changes in response to IL13 signaling were due to increased cytokine expression and not altered receptor levels, we analyzed our bulk and scRNA-seq data sets for the IL13 receptors Il13ra1 and Il4ra, and the inhibitory receptor Il13ra2. Comparison of receptor expression between Spry2ΔIE mice and littermate controls showed no significant differences (Figure 4D). Analysis of receptor expression in identified epithelial clusters showed expression of Il13ra1 and Il4ra in the colonic epithelium, with appreciable expression in DCS cells and other lineages, and no expression of Il13ra2 in epithelial cells (Figure 4E). This analysis showed expression of receptors for IL13 in crypt base cells, consistent with other reports,20 and suggests that IL13 signaling is being driven by increased cytokine level and not by altered receptor expression.
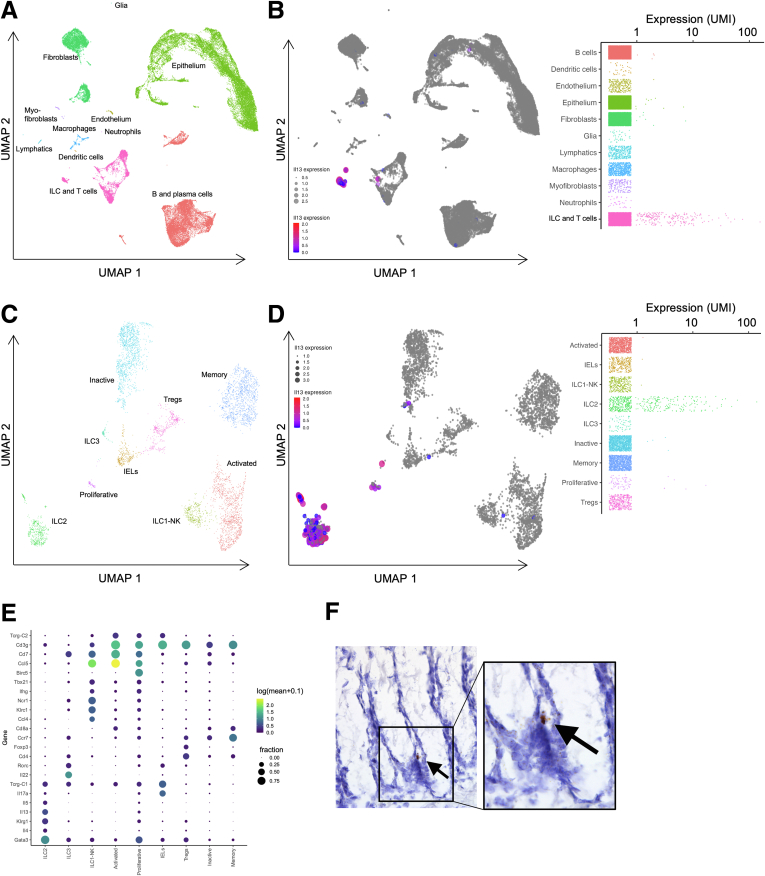
IL13 expression has been reported throughout the body in several immune cell populations besides ILC2s, raising the possibility that there could be multiple sources of IL13 in the colon.21,22 To determine if there were other likely sources of IL13, we analyzed our single-cell data set for expression among the recovered epithelial and stromal cell types. At the broadest level of clustering, this analysis showed almost no Il13 expression in epithelial or nonimmune mesenchymal cell populations (Figure 5A and B). The expression appeared highly specific to a subcategory of immune cells (Figure 5B); subsequent subclustering of this population defined this subcategory to represent the ILC2 population (Figure 5C and D). Thus, Il13 expression in the colon is largely restricted to ILC2 cells, with minimal expression observed in other colonic lymphocyte populations (Figure 5D and E). We previously showed that IL13-expressing cells can be detected along the length of the crypt.8 By in situ hybridization, we further show here that Il13+ cells are detectable in proximity to the crypt base (Figure 5F). Together these data show that ILC2s are the predominant source of IL13 in the murine colonic mucosa and locationally positioned to modify stem cell behavior and/or DCS cell development.
Figure 5.
ILC2s are the predominant source of IL13 in the colonic mucosa. (A) Uniform manifold approximation and projection (UMAP) plot of colonic epithelial and stromal cells subjected to scRNA-seq. (B) UMAP and population expression analysis showing Il13 expression largely restricted to the ILC and T-lymphocyte cluster. (C) Subclustering of the ILC and T-lymphocyte population shown by UMAP. (D) UMAP and population expression analysis showing Il13 expression is expressed predominantly in the ILC2 population, with minimal expression in other cell types. (E) Expression intensity dot map representing gene expression based on the fraction of cells that were positive and abundance of gene expression within each cluster in the ILC and T-lymphocyte subcluster. (F) In situ hybridization for Il13+ cells in the colonic epithelium. IEL, intraepithelial lymphocyte; NK, natural killer; UMI, unique molecular identifier.
IL13 Regulates Expression of DCS Cells In Vivo and In Vitro
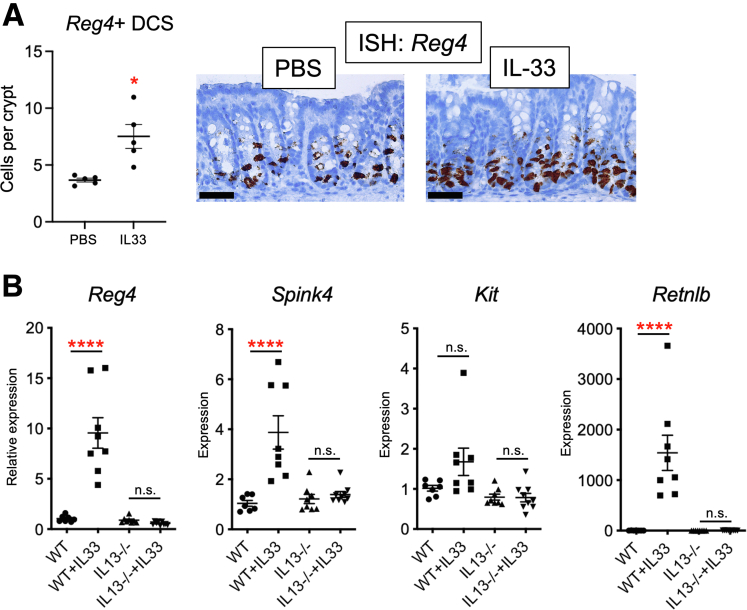
We and others previously showed that IL13 drives secretory lineage differentiation in the intestinal epithelium.8,23, 24, 25 In the colon, specifically, an epithelial–stromal IL33/IL13 circuit drives tuft and goblet cell expansion in Spry2ΔIE mice: the loss of epithelial Spry2 drives increased epithelial expression of IL33, thereby recruiting IL13-expressing ILC2s to mediate tuft and goblet cell differentiation.8 To determine whether this circuit contributes to DCS cell differentiation, we injected wild-type mice with IL33 for 4 days. Injected mice showed a doubling in the number of Reg4+ DCS cells (Figure 6A). Furthermore, IL33-injected wild-type mice showed increased colonic expression of the DCS cell markers Reg4, Spink4, and Retnlb (Figure 6B). Importantly, these effects were lost in IL13-/- mice. Thus, DCS cell population expansion can be driven by IL33 in vivo but requires IL13 signaling.
Figure 6.
The IL33/IL13 circuit induces expansion of the DCS cell population. (A) The IL33/IL13 axis was driven by 4 daily injections of 0.4 ug IL33. In situ hybridization for the DCS cell marker Reg4 was performed on distal colons showing increased numbers of Reg4+ cells in IL33-injected mice. n = 5 mice per group. Scale bar: 50 μm. (B) To test the requirement for IL13 in vivo, expression of DCS cell markers Reg4, Spink4, Kit, and Retnlb expression levels were quantified by quantitative polymerase chain reaction in colonic tissue of wild-type or IL13-/- mice injected with IL33. N = 7–9 mice per condition. Data were analyzed by 2-way analysis of variance with the Tukey post hoc test or 2-tailed Student t test. ∗P < .05, ∗∗∗∗P < .0001. ISH, in situ hybridization; PBS, phosphate-buffered saline; WT, wild-type.
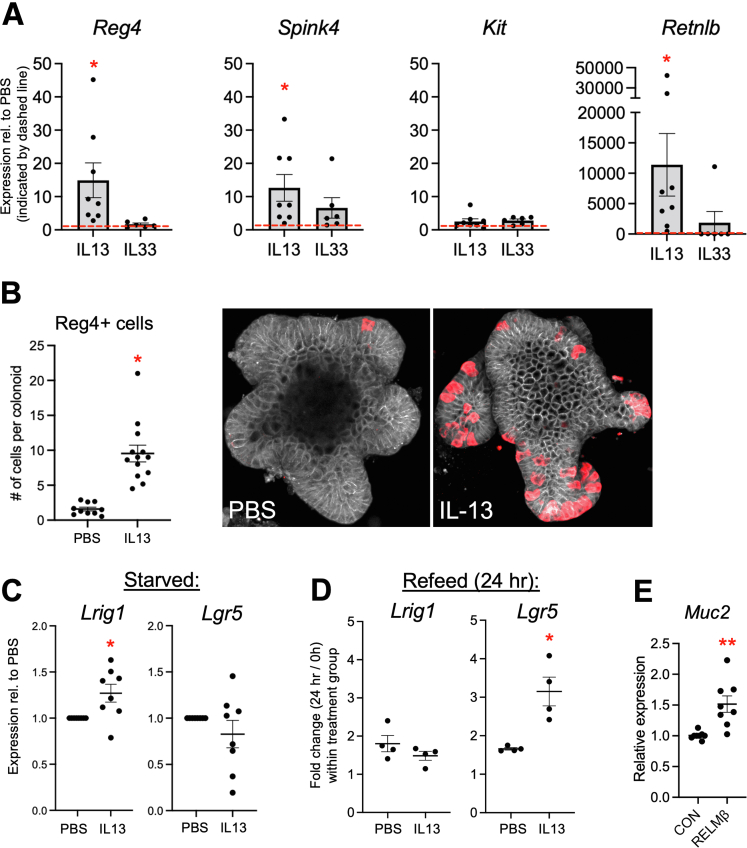
To further model this circuit in vitro, we treated colonic epithelial organoid (colonoid) cultures with either IL33 or IL13. After 24 hours, IL13 promoted a robust >10-fold induction of Reg4 and a >10-fold induction in Spink4 in colonoids (Figure 7A). Interestingly, this treatment caused a more than 10,000-fold induction in Retnlb. This suggests that IL13 may drive RELMβ expression in these cells beyond the stochiometric levels that would be expected by expanded DCS cell numbers alone, possibly indicating an activation state with increased expression per cell. In contrast, IL33 had no significant effects in colonoids. This shows that IL33 promotion of DCS cell development in vivo requires recruitment of a nonepithelial cell type and is mediated primarily by IL13, similar to the role of these cytokines in tuft and goblet cell differentiation.8,23 To test if the IL13-mediated increases in gene expression represented an expanded DCS cell population in this model, we immunostained colonoids for Reg4. Consistent with increased cell marker expression, IL13-treated colonoids showed a significant increase in the number of Reg4+ cells (Figure 7B).
Figure 7.
IL13 drives the DCS cell population expansion in colonoids. (A) Expression levels of DCS cell markers Reg4, Spink4, and Kit, and the HDP Retnlb were quantified in murine colonoids treated with 10 ng/mL IL13 or 100 ng/mL IL33 for 24 hours (n = 6–8 cultures per condition). Dashed line represents expression in phosphate-buffered saline (PBS) control. (B) Colonoids treated with 10 ng/mL IL13 were immunostained for Reg4 (red) and E-cadherin (white) and quantified to assess DCS cell numbers. n = 10–13 cultures per experiment. Each data point represents the average of an individual culture in an independent experiment. (C) Colonoids were primed for 24 hours with PBS or IL13 (10 ng/mL) to induce DCS cell development, then starved in growth factor–deficient culture media for 48 hours. Cultures were analyzed for expression of stem cell markers Lrig1 and Lgr5. (D) PBS or IL13 primed (24 h) and starved (48 h) cultures were re-fed with growth factor–replete media for 24 hours and analyzed by quantitative polymerase chain reaction (qPCR) for Lrig1 and Lgr5 induction. (E) Colonoids treated with RELMβ (100 ng/mL, 24 h) were analyzed by qPCR. Data were analyzed by 2-way analysis of variance with the Tukey post hoc test or 2-tailed Student t test. ∗P < .05, ∗∗P < .01. CON, control.
Because DCS cells reside at the base of the crypt, one putative role of these cells is maintenance of the stem cell niche.1,2 To test whether the IL13-mediated expansion of the DCS cell population could functionally protect this niche, we performed a stem cell maintenance and recovery assay during a challenge represented by the withdrawal of exogenous stem cell growth factors. Loss of leucine rich repeat containing G protein-coupled receptor 5 (Lgr5) positive stem cells and reduced expression of the progenitor marker Lrig1 occurs in various models of colonic injury and disease, and the restoration of these markers is associated with epithelial recovery.26 We primed colonoids with IL13 to increase DCS cell numbers, and then starved cultures of stem cell growth media for 48 hours. In IL13-primed cultures, the stem and progenitor cell marker Lrig1 was maintained at higher levels compared with unprimed cultures after 48 hours of challenge, suggesting that DCS cells contribute to protection of Lrig1+ cells during growth factor deprivation (Figure 7C). Furthermore, although Lgr5 was not preserved by IL13 in starved cultures, after replenishment of stem cell growth media for 24 hours we observed a more robust (3.1- vs 1.7-fold) recovery of Lgr5 (Figure 7D) in IL13-primed cultures vs unprimed cultures. These results are consistent with the previous finding that Lrig1 and Lgr5 levels are preserved in Spry2ΔIE mice subjected to DSS colitis.8 Because DCS cell ablation is associated with altered colonic secretory cells1 we next tested whether DCS cell–derived product could function in driving goblet cell activity by treating colonoids with RELMβ and assaying for Muc2. After 24 hours, RELMβ-treated cultures showed a significant increase in Muc2 expression compared with controls (Figure 7E), similar to previous in vitro findings.27 Together, these results support functional roles for DCS cells in the maintenance of the stem cell niche and regulation of colonic epithelial secretory cells.
Discussion
DCS cells are a crucial component of the colonic epithelium and are necessary for maintaining tissue health.1,2 They produce stem cell maintenance factors, and, as shown in our results, they also produce HDPs (Figure 2). Ablation of DCS cells leads to a loss of goblet cells and degradation of the epithelium.1 The number of DCS cells and other secretory lineages vary in response to modulation of growth factor signaling, showing that tissue-specific and microenvironment cues control their abundance,1,28, 29, 30 but very little is known about what signals regulate the number and function of these cells.
Here, we show that IL13, increased in mice with intestinal epithelial–specific deletion of the intracellular signaling regulator Sprouty2,8 promotes expansion of the DCS cell population in the colon. We previously reported that Sprouty2 down-regulation is an inflammation-responsive regulatory mechanism driving colonic tuft and goblet cell development via an epithelial–stromal IL33/IL13 signaling loop, and Sprouty2 loss protected mice against acute colitis.8 However, whether Sprouty2 and its actions on immune signaling regulate DCS cells remained unknown. Using the intestinal-specific Sprouty2 deletion mouse as a model of increased ILC2 activity, we show that IL13 is sourced from ILC2s and controls the census of DCS cells. Furthermore, short-circuit activation of the IL33/IL13 epithelial–stromal loop by injection of IL33 into mice or IL13 treatment in colonoids induces expression of DCS cell markers Reg4 and Spink4 and increases the number of Reg4+ DCS cells in the colon.
Immune signaling is known to regulate epithelial cell lineage allocation and function. In parasitic infections, epithelial production of IL33 and IL25 is enhanced to both recruit and activate IL13-expressing ILC2s.31,32 Increased IL13 drives secretory cell hyperplasia23,25 and promotes maintenance of colonic stem cells.20 In the colon, loss of Sprouty2 acts as an epithelial switch to drive production of IL33,8 leading to increased numbers of IL13-expressing ILC2s in the colon8 and an activated phenotype of these cells (Figure 4). Here, we have used the Spry2ΔIE mouse model to investigate the role of IL33 and IL13 signaling in DCS cell development. We found that IL33 alone is not sufficient to induce DCS cell marker expression in the epithelium, but requires IL13, which is induced by IL33 (Figures 6 and 7). Further studies with IL13-/- mice and colonoids confirmed that IL13 mediates the IL33 induction of DCS cell markers (Figures 6 and 7). Interestingly, recent work identified a fundamental role for cytokine signaling in maintaining homeostatic numbers of secretory cells in the human small intestine.33 Further work will be needed to determine if cytokine signaling serves a similar function in maintaining the homeostatic DCS cell balance in the colon, and how a complex cytokine milieu interacts to drive precise epithelial adaptations to maintain health.
The role of DCS cells in the colon is incompletely defined. These cells initially were thought to solely recapitulate the functions of Paneth cells, which reside at a similar crypt base position in the small intestine. However, because the environment of the small intestine and colon are markedly different and their epithelia face different microbial and tissue conditions (eg, pH, oxygen, metabolite levels), it is likely that a distinct set of specialized cells are required to maintain tissue homeostasis and health. In support of this, transcriptomic profiling by Sasaki et al1 found that DCS cells make up a discrete population that shares partial similarity to both intestinal goblet and Paneth cells at the gene expression level, but also has unique distinct properties. Furthermore, a single-cell analysis of the colon found that DCS cells have a similar gene expression profile with colonic secretory progenitors and goblet cells, consistent with our analysis.34 Taken together with our transcriptomic work, these findings suggest that DCS cells are a distinct cell type that develop from a common secretory progenitor in the colon. Because ablation of these cells impairs the stem cell niche and leads to loss of goblet cells,1 future studies will be necessary to understand their role and developmental trajectory in injury and disease. Furthermore, although some studies have described DCS cells as crypt base goblet cells, our data suggest a unique transcriptional profile for this population that is both functionally and developmentally distinct from goblet cells. Additional work will be needed to dissect whether multiple populations of secretory cells with distinct functions are present at the colonic crypt base2,35,36 and whether these cell types change along the length of the colon.
In both in vitro and in vivo experiments, we found that Kit expression is minimally responsive to cytokine treatment (Figures 6 and 7). This is consistent with our bulk RNA-seq data showing Kit is not increased significantly in Spry2ΔIE mice despite increased IL13 and increased numbers of Reg4+ DCS cells (Figure 1). Our single-cell expression data suggest that Kit is expressed more broadly in the epithelium than other molecules enriched in DCS cells (eg, Reg4, Spink4, and Agr2), despite the earlier identification of DCS cells as a Kit+ lineage. Additional work clarifying whether Kit marks a population of crypt secretory progenitors distinct from Reg4+ DCS cells, and the phenotypic equivalents of these discrete cellular populations in human colon, will be an important area to clarify in future work.
In addition to identifying an IL13-mediated mechanism regulating DCS cells, our study suggests new specialized functions of this cell type. By transcriptomic profiling of colonic tissue and single cells, we have identified a previously unappreciated role for DCS cells at homeostasis in selective production of the HDP RELMβ (Figure 2). HDPs are produced throughout the body in an organ- and cell-specific manner. Expression of the resistin family of HDPs has been shown previously in the colon, and here we advance this knowledge by performing an unbiased transcriptomic analysis for expression of multiple classes of HDPs. Using bulk RNA-seq from colonic tissue, we performed a broad evaluation of HDP expression and found expression of several HDPs (Restn, Retnla, Retnlb, Camp, Defb1, Defb42, and Defb45) that we further located in distinct cell populations in the colonic epithelium using scRNA-seq (Figure 3). Surprisingly, RELMβ was the only HDP that localized specifically to DCS cells. These results suggest that DCS cells may serve as a reservoir of RELMβ in the absence of disease. Consistent with our finding that Spry2ΔIE mice have higher numbers of DCS cells, these mice also showed increased expression of RELMβ (Figure 2), further supporting the hypothesis that Sprouty2 serves as a tissue environmental sensor to rapidly control the census of mature cell types depending on microenvironment conditions (eg, infection, injury, or inflammation). Interestingly, our data show that RELMβ can regulate the functional goblet cell marker Muc2 in colonoids (Figure 7), thus implying that dynamic regulation of DCS cells subsequently may affect additional secretory cell populations.
RELMβ expression in colonic goblet cells has been reported during inflammation.5,37 Here, we show by transcriptomic profiling that RELMβ marks DCS cells, but not goblet cells, at homeostasis (Figure 2). Consistent with previous findings,38 this production occurs even in the absence of colitis, thus providing evidence that DCS cells play roles in homeostasis outside of maintenance of the stem cell niche. However, because the tissue environmental requirements for HDPs may differ along the length of the colon, future work is necessary to understand discrete locational and functional roles of DCS cells in distinct regions of the intestinal tract, as well as identifying how these cells respond to overt injury and disease.
In summary, our findings suggest that DCS cell numbers are controlled by an epithelial-immune circuit. IL13 signaling, activated by down-regulation of epithelial Sprouty2 and an increase in ILC2s, drives DCS cell population expansion in the colon. The increase in DCS cell numbers likely serves as a protective response to damage or inflammation because these cells and their secreted products can limit pathogen invasion or alter the microbiota, regulate epithelial secretory cells, and promote maintenance or regeneration of the stem cell niche.
Materials and Methods
Animal Experiments
Mice with an intestinal epithelial–specific deletion of Sprouty2 (VillinCre;Spry2flox/flox; herein termed Spry2ΔIE) were generated by crossing VillinCre animals with mice harboring loxP-flanked Spry2 (Spry2flox/flox; herein termed Spry2FF).8 Male and female Spry2ΔIE and Spry2FF littermate controls aged 7–8 weeks were used for experiments. IL13-/- mice and controls aged 6–12 weeks were given daily intraperitoneal injections of 0.4 μg recombinant IL33 for 4 days as previously described.23
RNA Sequencing
To generate a single-cell suspension, colonic mucosal peelings were isolated from 4 Spry2ΔIE and 4 Spry2FF littermate controls, and digested for 30 minutes at 37°C in 100 mL Dulbecco’s modified Eagle medium with 2% heat-inactivated fetal bovine serum, 0.2 mg/mL dispase II (D4693; Sigma), 2 mg/mL collagenase D (#11088882001; Roche, Indianapolis, IN), and 0.2 mg/mL DNase I (DN25; Sigma).39 scRNA-seq was performed on this population using the Chromium Single-Cell System (10× Genomics) and sequencing on a HiSeq flowcell to a total of 1.75 billion reads with a minimum of 20,000 reads per cell and are publicly available (GSE196803). Samples were processed using CellRanger and quality control filtered so that cells with fewer than 100 unique molecular identifier reads were discarded. Cell populations were clustered in monocle3 using the Leiden clustering algorithm applied to uniform manifold approximation and projection of the top 100 principal coordinates of dimensionally reduced data.40 Gene enrichment in identified populations was performed with the marker_test_res() function. Dot graphs and violin plots were generated in R. Bulk tissue colonic RNA-seq from Spry2ΔIE and Spry2FF littermate controls was generated previously8 and publicly available (GSE160854).
Real-Time Polymerase Chain Reaction
RNA from cells and tissue was collected using on-column RNA isolation and purification (Omega Bio-tek), and complementary DNA was generated with a high-capacity complementary DNA reverse-transcriptase kit (4368814; Applied Biosystems). Quantitative analysis of expression was performed using TaqMan assays (Hprt [Mm03024075_m1], Reg4 [Mm00471115_m1], Kit [Mm00445212_m1], Spink4 [Mm00803437_m1], Agr2 [Mm00507853_m1], Spdef [Mm00600221_m1], Muc2 [Mm01276696_m1], Lgr5 [Mm00438890_m1], Lrig1 [Mm456116_m1], and Retnlb [Mm00445845_m1]) on an Applied Biosystems StepOne thermocycler. Fold change was calculated using the 2-ΔΔCt method. Results are expressed as the average fold change in gene expression relative to control or nontreatment group using Hprt as the reference gene.
RNAScope In Situ Hybridization
Distal colonic sections (cut at 5 μm from tissue fixed with 4% formaldehyde overnight and paraffin-embedded), were probed using RNAScope probes Mm-Reg4 (#409601; Advanced Cell Diagnostics) and Mm-Il13 (#312291; Advanced Cell Diagnostics) using the RNAScope 2.5 HD Detection system (#322310; Advanced Cell Diagnostics) according to the manufacturer-provided protocols.
Immunofluorescence Staining
Fixed colonic sections were blocked with 10% goat serum for 1 hour at room temperature followed by incubation with primary antibody against RELMβ (#PA1-1047, 1:100; Invitrogen) and E-cadherin (#610181, 1:500; BD Biosciences) overnight at 4°C. Sections were washed and incubated with secondary anti-mouse Alexa Fluor–488 (#A11029, 1:1000; Life Technologies) or anti-rabbit Alexa Fluor-546 (#A11035, 1:2000; Life Technologies) overnight at 4°C, followed by mounting with Diamond Prolong Glass Mountant containing NucBlue (P36981; Invitrogen). Imaging was performed on a Leica DM4000B fluorescence microscope using Leica Application Suite X software.
Cell and Organoid Culture
Colonoids were generated from mouse colon using modified previously established protocols.41 Briefly, crypts were isolated by EDTA chelation and mechanical dissociation and embedded into Matrigel (BD Biosciences), and then overlaid with murine Intesticult growth media (Stem Cell Technologies). Cultures were passaged every 7–10 days by mechanical trituration and shearing of colonoids before re-embedding. Passaged cultures were starved in basal medium without growth factors (advanced Dulbecco’s modified Eagle medium/F12 supplemented with 2 mmol/L GlutaMax (Thermo Fisher), 10 mmol/L HEPES, 1× penicillin-streptomycin, 1× N2 supplement, and 1× B27 supplement) overnight, treated with IL33 (#210-33; Peprotech) or IL13 (#210-13; Peprotech), and collected at 24 hours. In some experiments, cultures were treated with RELMβ (76238; Bio-techne) for 24 hours. For imaging, colonoids grown on chambered coverglass slides (155411; Nunc) were fixed with 4% paraformaldehyde at room temperature, permeabilized with phosphate-buffered saline + 0.1% Triton X-100, and blocked with 10% goat serum. Cultures then were incubated for 48 hours with primary antibody against Reg4 (#80249, 1:500; Sino Biological) and E-cadherin (#610181, 1:200; BD Biosciences), and incubated overnight with secondary anti-mouse Alexa Fluor–488 (#A11029, 1:200; Life Technologies) or anti-rabbit Alexa Fluor–546 (#A11035, 1:200; Life Technologies) overnight at 4°C. Imaging was performed on a Zeiss LSM 700 using Zen 2009 software.
Statistics
Statistical analyses and plots were generated using Prism 8 (GraphPad Software). Means ± SEM are depicted in dot and bar graphs. A 2-tailed Student t test or analysis of variance with the Tukey post hoc test to correct for multiple comparisons was used to determine statistical differences, as appropriate. Statistical significance was set at P < .05, and is indicated in the figure legends.
Study Approval
All animal use was approved and monitored by the Children’s Hospital Los Angeles Institutional Animal Care and Use Committee (Animal Welfare Assurance #A3276-01) or the Cincinnati Children’s Hospital Medical Center Institutional Animal Care and Use Committee (Animal Welfare Assurance #A3108-01). Mice were housed under standard conditions with ad libitum water and chow access in the Association for Assessment and Accreditation of Laboratory Animal Care–accredited animal care facilities at Children’s Hospital Los Angeles or Cincinnati Children’s Hospital Medical Center.
Acknowledgments
The authors thank Kaya Johnson (Marlborough High School) for assistance in curation of RNA sequencing data, Dr Esteban Fernandez and the Cellular Imaging Core (Children’s Hospital Los Angeles), and Long Hung and the Single Cell, Sequencing, and CyTOF Core Laboratory (Children’s Hospital Los Angeles) for their support of this work.
CRediT Authorship Contributions
Michael A Schumacher, PhD (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Funding acquisition: Supporting; Investigation: Lead; Writing – original draft: Lead; Writing – review & editing: Lead)
Cambrian Y Liu, PhD (Data curation: Equal; Formal analysis: Equal; Investigation: Equal; Writing – review & editing: Equal)
Kay Katada, BS (Formal analysis: Equal; Investigation: Equal; Writing – review & editing: Equal)
Megan H Thai, BS (Investigation: Equal; Writing – review & editing: Equal)
Jonathan J Hsieh, BS (Investigation: Equal; Writing – review & editing: Equal)
Britany J Hansten (Investigation: Equal; Writing – review & editing: Equal)
Amanda Waddell, PhD (Investigation: Equal; Writing – review & editing: Equal)
Michael J Rosen, MD PhD (Funding acquisition: Equal; Investigation: Equal; Writing – review & editing: Equal)
Mark R Frey, PhD (Conceptualization: Lead; Formal analysis: Equal; Funding acquisition: Lead; Investigation: Lead; Writing – original draft: Equal; Writing – review & editing: Equal; Co-corresponding author: Lead)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by Career Development Awards from the Crohn’s and Colitis Foundation (M.A.S., C.Y.L.), a Research Fellowship Award from the Crohn’s and Colitis Foundation (A.W.), a Research Career Development Award from The Saban Research Institute at Children’s Hospital Los Angeles (M.A.S.), a core facility pilot award from Children’s Hospital Los Angeles (M.A.S.), funding from CURE for IBD (M.J.R.), and National Institutes of Health grants K01DK131390 (M.A.S.), P30DK042086 (C.Y.L.), R01DK095004 and R01DK119694 (M.R.F.), and R01DK117119 (M.J.R.).
Data Availability Statement Single-cell RNA sequencing data are publicly available (GSE196803).
Contributor Information
Michael A. Schumacher, Email: mschumacher@chla.usc.edu.
Mark R. Frey, Email: mfrey@chla.usc.edu.
References
- 1.Sasaki N., Sachs N., Wiebrands K., et al. Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc Natl Acad Sci U S A. 2016;113:E5399–E5407. doi: 10.1073/pnas.1607327113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothenberg M.E., Nusse Y., Kalisky T., et al. Identification of a cKit(+) colonic crypt base secretory cell that supports Lgr5(+) stem cells in mice. Gastroenterology. 2012;142:1195–1205.e6. doi: 10.1053/j.gastro.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotarsky K., Sitnik K.M., Stenstad H., et al. A novel role for constitutively expressed epithelial-derived chemokines as antibacterial peptides in the intestinal mucosa. Mucosal Immunol. 2010;3:40–48. doi: 10.1038/mi.2009.115. [DOI] [PubMed] [Google Scholar]
- 4.Munitz A., Waddell A., Seidu L., et al. Resistin-like molecule α enhances myeloid cell activation and promotes colitis. J Allergy Clin Immunol. 2008;122:1200–1207.e1. doi: 10.1016/j.jaci.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergstrom K.S.B., Morampudi V., Chan J.M., et al. Goblet cell derived RELM-β recruits CD4+ T cells during infectious colitis to promote protective intestinal epithelial cell proliferation. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morampudi V., Dalwadi U., Bhinder G., et al. The goblet cell-derived mediator RELM-β drives spontaneous colitis in Muc2-deficient mice by promoting commensal microbial dysbiosis. Mucosal Immunol. 2016;9:1218–1233. doi: 10.1038/mi.2015.140. [DOI] [PubMed] [Google Scholar]
- 7.Hogan S.P., Seidu L., Blanchard C., et al. Resistin-like molecule β regulates innate colonic function: barrier integrity and inflammation susceptibility. J Allergy Clin Immunol. 2006;118:257–268. doi: 10.1016/j.jaci.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumacher M.A., Hsieh J.J., Liu C.Y., et al. Sprouty2 limits intestinal tuft and goblet cell numbers through GSK3β-mediated restriction of epithelial IL-33. Nat Commun. 2021;12:836. doi: 10.1038/s41467-021-21113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Artis D., Wang M.L., Keilbaugh S.A., et al. RELM/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci U S A. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blyth G.A.D., Connors L., Fodor C., Cobo E.R. The network of colonic host defense peptides as an innate immune defense against enteropathogenic bacteria. Front Immunol. 2020;11:965. doi: 10.3389/fimmu.2020.00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu B., Yang L., Zhu X., et al. Yeats4 drives ILC lineage commitment via activation of Lmo4 transcription. J Exp Med. 2019;216:2653–2668. doi: 10.1084/jem.20182363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vivier E., van de Pavert S.A., Cooper M.D., Belz G.T. The evolution of innate lymphoid cells. Nat Immunol. 2016;17:790–794. doi: 10.1038/ni.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L., Tang J., Yang X., et al. TGF-β induces ST2 and programs ILC2 development. Nat Commun. 2020;11:35. doi: 10.1038/s41467-019-13734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallrapp A., Burkett P.R., Riesenfeld S.J., et al. Calcitonin gene-related peptide negatively regulates alarmin-driven type 2 innate lymphoid cell responses. Immunity. 2019;51:709–723.e6. doi: 10.1016/j.immuni.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagasawa M., Heesters B.A., Kradolfer C.M.A., et al. KLRG1 and NKp46 discriminate subpopulations of human CD117+CRTH2− ILCs biased toward ILC2 or ILC3. J Exp Med. 2019;216:1762–1776. doi: 10.1084/jem.20190490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sudo T., Motomura Y., Okuzaki D., et al. Group 2 innate lymphoid cells support hematopoietic recovery under stress conditions. J Exp Med. 2021;218 doi: 10.1084/jem.20200817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudo F., Ikutani M., Seki Y., et al. Interferon-γ constrains cytokine production of group 2 innate lymphoid cells. Immunology. 2016;147:21–29. doi: 10.1111/imm.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh P.B., Pua H.H., Happ H.C., et al. MicroRNA regulation of type 2 innate lymphoid cell homeostasis and function in allergic inflammation. J Exp Med. 2017;214:3627–3643. doi: 10.1084/jem.20170545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzurana L., Czarnewski P., Jonsson V., et al. Tissue-specific transcriptional imprinting and heterogeneity in human innate lymphoid cells revealed by full-length single-cell RNA-sequencing. Cell Res. 2021;31:554–568. doi: 10.1038/s41422-020-00445-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu P., Zhu X., Wu J., et al. IL-13 secreted by ILC2s promotes the self-renewal of intestinal stem cells through circular RNA circPan3. Nat Immunol. 2019;20:183–194. doi: 10.1038/s41590-018-0297-6. [DOI] [PubMed] [Google Scholar]
- 21.McKenzie G.J., Bancroft A., Grencis R.K., McKenzie A.N. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol. 1998;8:339–342. doi: 10.1016/s0960-9822(98)70134-4. [DOI] [PubMed] [Google Scholar]
- 22.Fuss I.J., Heller F., Boirivant M., et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waddell A., Vallance J.E., Hummel A., et al. IL-33 induces murine intestinal goblet cell differentiation indirectly via innate lymphoid cell il-13 secretion. J Immunol. 2019;202:598–607. doi: 10.4049/jimmunol.1800292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marillier R.G., Michels C., Smith E.M., et al. IL-4/IL-13 independent goblet cell hyperplasia in experimental helminth infections. BMC Immunol. 2008;9:11. doi: 10.1186/1471-2172-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manocha M., Shajib M.S., Rahman M.M., et al. IL-13-mediated immunological control of enterochromaffin cell hyperplasia and serotonin production in the gut. Mucosal Immunol. 2013;6:146–155. doi: 10.1038/mi.2012.58. [DOI] [PubMed] [Google Scholar]
- 26.Girish N., Liu C.Y., Gadeock S., et al. Persistence of Lgr5+ colonic epithelial stem cells in mouse models of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2021;321:G308–G324. doi: 10.1152/ajpgi.00248.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krimi R.B., Kotelevets L., Dubuquoy L., et al. Resistin-like molecule β regulates intestinal mucous secretion and curtails TNBS-induced colitis in mice. Inflamm Bowel Dis. 2008;14:931–941. doi: 10.1002/ibd.20420. [DOI] [PubMed] [Google Scholar]
- 28.Schumacher M.A., Danopoulos S., Al Alam D., Frey M.R. Growth factors in the intestinal tract. Physiology of the Gastrointestinal Tract. 6th ed. Elsevier, Inc; 2018. [Google Scholar]
- 29.Almohazey D., Lo Y.-H., Vossler C.V., et al. The ErbB3 receptor tyrosine kinase negatively regulates Paneth cells by PI3K-dependent suppression of Atoh1. Cell Death Differ. 2017;24:855–865. doi: 10.1038/cdd.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heuberger J., Kosel F., Qi J., et al. Shp2/MAPK signaling controls goblet/Paneth cell fate decisions in the intestine. Proc Natl Acad Sci U S A. 2014;111:3472–3477. doi: 10.1073/pnas.1309342111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Moltke J., Ji M., Liang H.-E., Locksley R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neill D.R., Wong S.H., Bellosi A., et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He G.-W., Lin L., DeMartino J., et al. Optimized human intestinal organoid model reveals interleukin-22-dependency of Paneth cell formation. Cell Stem Cell. 2022;29:1333–1345.e6. doi: 10.1016/j.stem.2022.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Habowski A.N., Flesher J.L., Bates J.M., et al. Transcriptomic and proteomic signatures of stemness and differentiation in the colon crypt. Commun Biol. 2020;3:453. doi: 10.1038/s42003-020-01181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou Q., Huang J., Ayansola H., et al. Intestinal stem cells and immune cell relationships: potential therapeutic targets for inflammatory bowel diseases. Front Immunol. 2021;11:3523. doi: 10.3389/fimmu.2020.623691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burclaff J., Bliton R.J., Breau K.A., et al. A proximal-to-distal survey of healthy adult human small intestine and colon epithelium by single-cell transcriptomics. Cell Mol Gastroenterol Hepatol. 2022;13:1554–1589. doi: 10.1016/j.jcmgh.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parmar N., Burrows K., Vornewald P.M., et al. Intestinal-epithelial LSD1 controls goblet cell maturation and effector responses required for gut immunity to bacterial and helminth infection. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steppan C.M., Brown E.J., Wright C.M., et al. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci U S A. 2001;98:502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schumacher M.A., Hedl M., Abraham C., et al. ErbB4 signaling stimulates pro-inflammatory macrophage apoptosis and limits colonic inflammation. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao J., Spielmann M., Qiu X., et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature. 2019;566:496–502. doi: 10.1038/s41586-019-0969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahe M.M., Aihara E., Schumacher M.A., et al. Establishment of gastrointestinal epithelial organoids. Curr Protoc Mouse Biol. 2013;3:217–240. doi: 10.1002/9780470942390.mo130179. [DOI] [PMC free article] [PubMed] [Google Scholar]