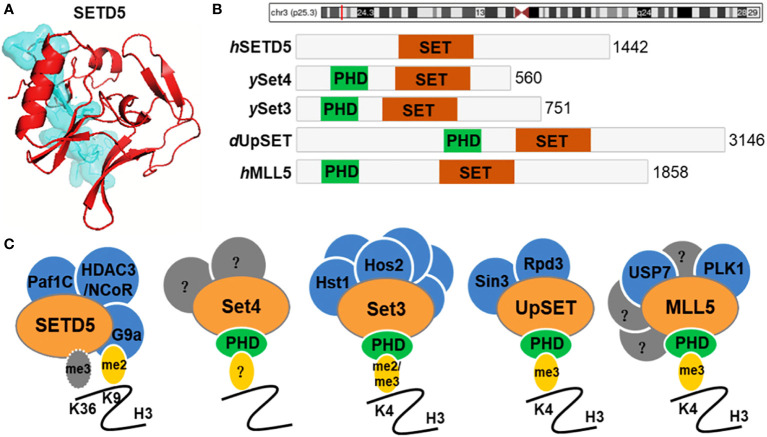
Figure 1.
SETD5 domain composition and homologue architecture. (A) Crystal structure of human SETD5 protein. (B) Schematic indicating the protein domain organization of human (h) SETD5 and MLL5, yeast (y) Set3 and Set4, and Drosophila (d) UpSET. SET domains are shown in red and PHD fingers are shown in green. The total number of amino acids is indicated for each protein. (C) SETD5 contains the SET domain and is annotated as a candidate protein of lysine methyltransferase, which methylates H3K36 residue. However, there is evidence that SETD5 lacks the methyltransferase activity but scaffolds the G9a/HDAC3 co-repressor complex, which couples methylation of H3K9 with deacetylation of this residue. Members of the Set3-Set4 SET domain subfamily are shown with known interacting partners and methyl-lysine binding activity of their PHD fingers. Known binding partners are shown in blue. Set4 is predicted to interact with other factors (shown in gray) that remain to be identified. MLL5 has known interactors, a subset of which are shown in blue, and other yet-to-be-determined factors are indicated in gray.