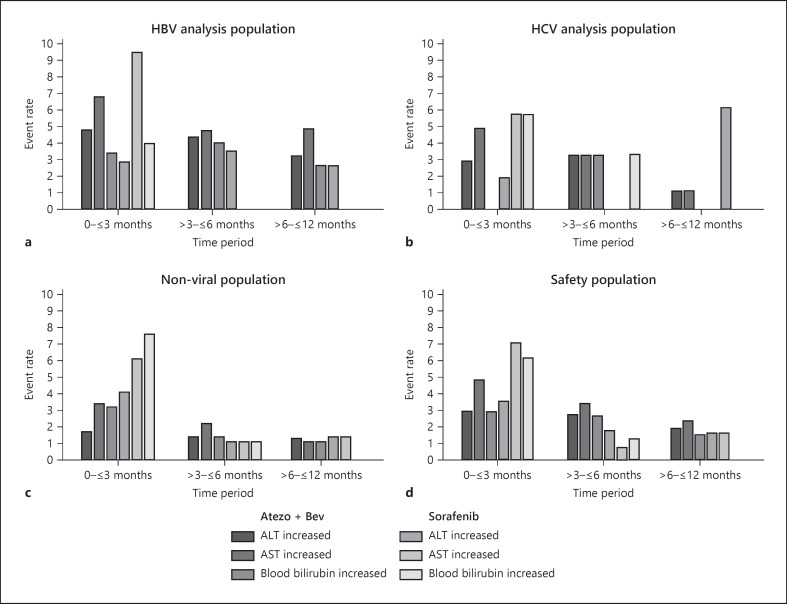
Fig. 1.
Exposure-adjusted hepatic adverse events by observation period. ALT increase, AST increase, and blood bilirubin increase in the (a) HBV (b) HCV (c) nonviral, and (d) safety populations. There were no ALT, AST, or blood bilirubin increased adverse events reported beyond 12 months. Multiple occurrences of the same AE in an individual are counted only once; rate is number of patients who experienced event over total exposure. ALT, alanine aminotransferase; AST, aspartate aminotransferase; atezo, atezolizumab; bev, bevacizumab.