Figure 3.
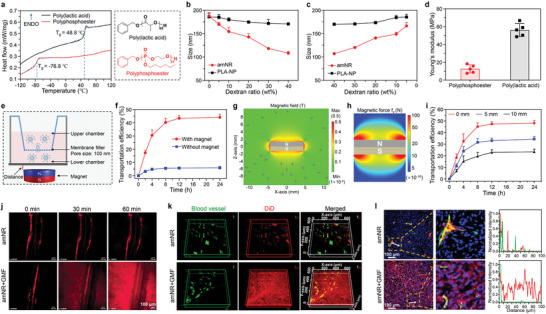
The distinctive deformability of the amNRs ensured their magnetic field‐driven deep penetration into tumor tissue. a) Differential scanning calorimetry (DSC) traces of polyphosphoester (PPE) and poly(lactic acid) (PLA). The glass transition temperatures (T gs) of PPE and PLA were −76.8 °C and 48.8 °C, respectively. The right panel shows the chemical structures of PPE and PLA. b,c) Hydrodynamic diameters of amNRs (PPE core) and PLA‐NPs (PLA core) in dextran solutions with different concentrations. d) The Young's modulus was determined by atomic force microscopy of PPE and PLA. e) Schematic illustration of the 100 nm filter membrane fixed Transwell system to evaluate the deformability of the amNRs under a GMF. f) Transportation efficiency of amNRs across a 100 nm membrane filter under a GMF. g) Representative finite element modeling of the magnetic field magnitude B plots for the 8 mm diameter and 3 mm thick NdFeB cylindrical magnet. h) Simulated distribution of the magnetic force on the amNRs in the z‐direction. The simulated magnetic force calculations were based on the method reported in the literature.[ 26 ] i) Transportation efficiency of amNRs across a 100 nm membrane filter at different distances from the cylindrical magnet. The distance was considered the length from the surface of the cylindrical magnet to the bottom of the Transwell. j) Real‐time intravital observation of the distribution of DiD‐labeled amNRs (red) in the dorsal skin fold window chamber tumor model after intravenous administration. k) Spatial distribution of DiD‐labeled amNRs (red) in tumor tissue. l) The intratumoral distribution of DiD‐labeled amNRs under a GMF. Blood vessels: green; the cellular nuclei: blue. The fluorescence colocalization analysis of DiD‐labeled amNRs (red line) and blood vessels (green line) was shown in the right section.
