Figure 4.
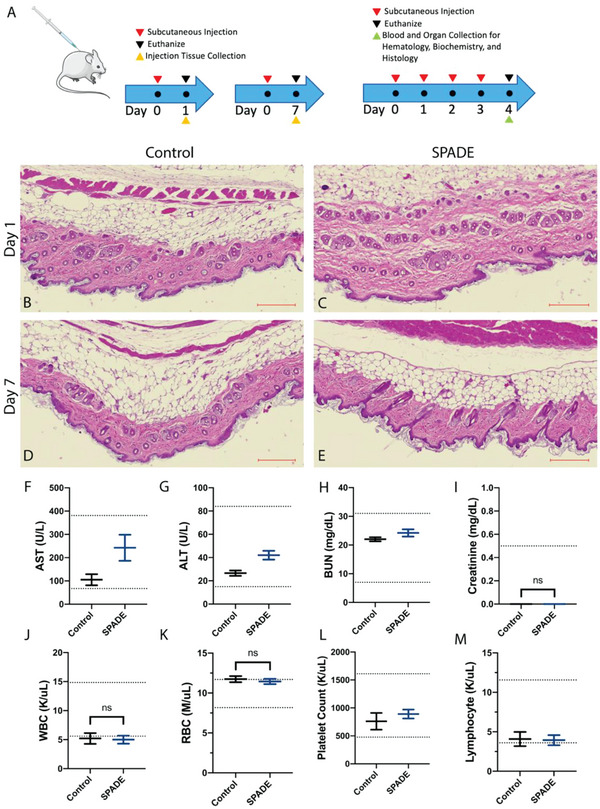
SPADE safety assessment. A) Safety study designs for SPADE versus control (saline) including injection site toxicity (left) and repeat dosing study (right). The studies incorporated subcutaneous injection (red arrow), injection site tissue collection (yellow arrow), blood and vital organ collection (green arrow), and euthanization (black arrow). B–E) Injection site H&E images for indicated formulation and time points. Scale bars, 200 µm. F) aspartate aminotransferase (AST) serum concentrations. G) Alanine aminotransferase (ALT) serum concentrations groups. H) Blood urea nitrogen (BUN) serum concentrations. I) Creatinine serum concentrations. J) White blood cell (WBC) counts. K) Red blood cell (RBC) counts. L) Platelet counts. M) Lymphocyte counts for control (n = 4) and SPADE (n = 5) treated groups. Dotted lines represent the expected range for metric of interest.[ 45 ] Statistical significance was determined with a t‐test. *p < 0.05. The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
