Figure 2. Grading of Recommendations Assessment, Development and Evaluation Summary of Findings Table Outlining Certainty of Evidence and Absolute Risks With Triplet Therapy (Columns) Compared With Other Treatments (Rows) in Overall Patient Population.
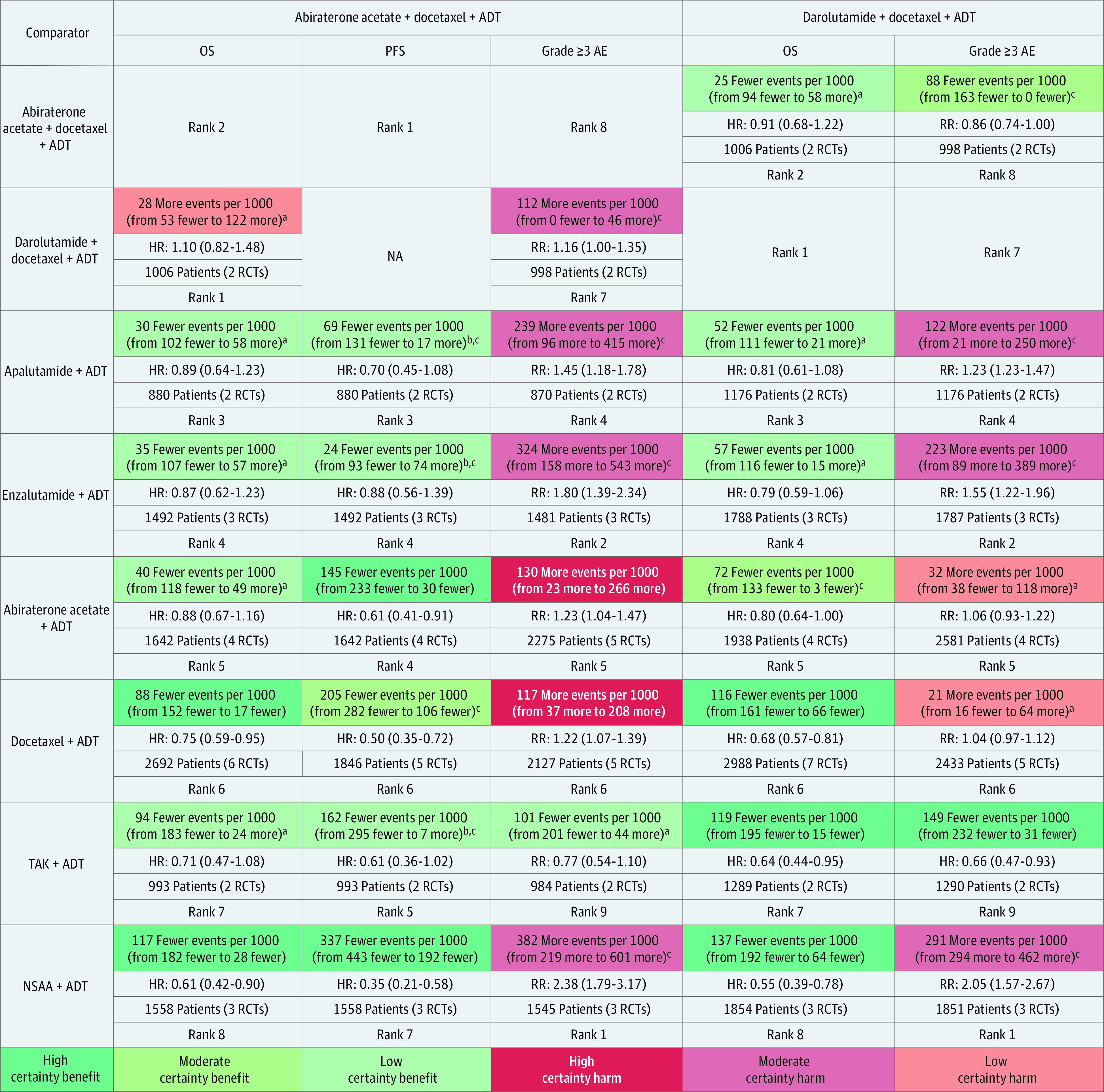
Summary of relative and absolute risks for mixed treatment comparisons derived from frequentist network meta-analysis using 4 levels of certainty: high (further research very unlikely to change confidence in estimate of effect), moderate (further research likely to have important association with confidence in estimate of effect and may change the estimate), low (further research very likely to have important association with confidence in estimate of effect and likely to change the estimate), and very low (very uncertain about the estimate). Values in cells indicate relative and absolute effect estimates for comparisons between triplet therapies (abiraterone and darolutamide triplet therapies) and other treatment options (androgen pathway inhibitor [API] doublet, docetaxel doublet, and androgen deprivation therapy [ADT]). Each comparison consists of absolute reduction in risk of events (upper cell) with triplet therapies compared with other treatments, relative effect hazard ratios (HRs; upper middle cell), sample size contributive to evidence (lower middle cell), and relative rank of treatment in a row (lower cell). AE indicates adverse event; NSAA, nonsteroidal antiandrogen; OS, overall survival; PFS, progression-free survival; RCTs, randomized clinical trials; RR, relative risk; and TAK, orteronel.
aRated down 2 levels owing to very serious imprecision, considering null effect and wide 95% CIs indicating both potential benefit and harm.
bRated down 1 level owing to serious imprecision, considering wide 95% CIs.
cRated down 1 level owing to serious indirectness in definition of PFS used across trials; PFS may be an advantageous end point for APIs owing to fixed dosing schedule of docetaxel compared with most API trials that used an indefinite dosing until disease progression.
