Abstract
Prior research suggests that fluvoxamine, a selective serotonin reuptake inhibitor (SSRI) used for the treatment of obsessive-compulsive disorder and major depressive disorder, could be repurposed against COVID-19. We undertook a prospective interventional open-label cohort study to evaluate the efficacy and tolerability of fluvoxamine among inpatients with laboratory-confirmed COVID-19 in Uganda. The main outcome was all-cause mortality. Secondary outcomes were hospital discharge and complete symptom resolution. We included 316 patients, of whom 94 received fluvoxamine in addition to standard care [median age, 60 years (IQR = 37.0); women, 52.2%]. Fluvoxamine use was significantly associated with reduced mortality [AHR = 0.32; 95% CI = 0.19–0.53; p < 0.001, NNT = 4.46] and with increased complete symptom resolution [AOR = 2.56; 95% CI = 1.53–5.51; p < 0.001, NNT = 4.44]. Sensitivity analyses yielded similar results. These effects did not significantly differ by clinical characteristic, including vaccination status. Among the 161 survivors, fluvoxamine was not significantly associated with time to hospital discharge [AHR 0.81, 95% CI (0.54–1.23), p = 0.32]. There was a trend toward greater side effects with fluvoxamine (7.45% versus 3.15%; SMD = 0.21; χ2 = 3.46, p = 0.06), most of which were light or mild in severity and none of which were serious. One hundred mg of fluvoxamine prescribed twice daily for 10 days was well tolerated and significantly associated with reduced mortality and with increased complete symptom resolution, without a significant increase in time to hospital discharge, among inpatients with COVID-19. Large-scale randomized trials are urgently needed to confirm these findings, especially for low- and middle-income countries, where access to vaccines and approved treatments against COVID-19 is limited.
Subject terms: Diseases, Physiology, Prognostic markers
Introduction
The SARS-CoV-2 pandemic has created a tremendous economic and health crisis worldwide [1–3] and has led to excess mortality, especially in low- and middle-income countries [4, 5]. Because a large portion of the world’s population is currently unvaccinated [6], effective treatments for COVID-19—especially those that can be administered orally, have good tolerability, low rates of medical contraindications [7, 8], and wide availability at low cost—are urgently needed to reduce COVID-19-related mortality and morbidity [9–11]. This is particularly important in low- and middle-income countries, where access to vaccines and approved treatments against COVID-19 is limited [9–11].
Prior research suggests that fluvoxamine, a selective serotonin reuptake inhibitor (SSRI) used for the treatment of obsessive-compulsive disorder and major depressive disorder [12], could be repurposed against COVID-19 [9–11, 13–16]. In the ambulatory setting, three studies, including two randomized, placebo-controlled trials (RCTs) and one nonrandomized open-label clinical study, found a significant association between the short-term use (10–15 days) of fluvoxamine prescribed at a daily dose between 100 and 300 mg and taken within 7 days of symptom onset and a reduced risk of clinical deterioration [17–19]. A prospective cohort study of patients admitted to intensive care units (ICUs) for COVID-19 also reported a significant association between the 15-day use of fluvoxamine prescribed at a daily dose of 300 mg and reduced mortality [20]. Conversely, an RCT of low-dose fluvoxamine [21] (i.e., 100 mg/day) among overweight or obese outpatients with COVID-19 showed no significant benefit on emergency department visits, hospitalizations or death. These findings suggest that the use of fluvoxamine, when prescribed at a daily dose of 200–300 mg, may improve the clinical outcomes of patients infected with SARS-CoV-2, including mortality, in both ambulatory and acute care settings.
The benefits of fluvoxamine as a treatment for COVID-19 are believed to stem from several mechanisms [13, 18, 19, 22–27]. They include antiviral and anti-inflammatory effects via functional inhibition of acid sphingomyelinase (FIASMA), immunomodulatory activity via sigma-1 receptor (S1R) agonism and non-S1R pathways (e.g., NF-κB, inflammasomes, TLR4, PPARγ), and mechanisms related to increased plasma melatonin levels, serotonin modulation, and antiplatelet activity. Specifically, before the COVID-19 pandemic, fluvoxamine showed anti-inflammatory properties in murine models of septic shock [28], which may be mediated by the S1R pathway of cytokine release [24, 25, 29, 30]. Another mechanism through which the beneficial effects of fluvoxamine could be exerted is the acid sphingomyelinase/ceramide (ASM) pathway [27, 31–33]. Inhibition of the ASM/ceramide system (called FIASMA) by specific antidepressants such as fluvoxamine or fluoxetine prevents infection of Vero E6 cells with SARS-CoV-2 [23]. The reconstitution of ceramides in cells treated with these specific antidepressants restores the infection [23]. Furthermore, inhibition of ASM in endothelial cells and the immune system may also result in anti-inflammatory effects [9]. These data were reinforced in several other studies. First, several observational cohort studies of patients with COVID-19 reported reduced deaths or use of mechanical ventilation in the acute care setting [33–37] and reduced risk of emergency department or hospital visits in the ambulatory setting [38] among those taking FIASMA antidepressants versus their counterparts. Second, preclinical studies have demonstrated the in vitro efficacy of several SSRI and non-SSRI FIASMA antidepressants against different variants of SARS-CoV-2 in human and nonhuman host cells [22, 39–45]. Finally, a recent work [46] supported the antiviral and anti-inflammatory properties of fluoxetine, an SSRI antidepressant with FIASMA properties, in a K18-hACE2 mouse model of SARS-CoV-2 infection and its in vitro antiviral activity against different variants of concern, including Omicron BA.5.
Due to limited access to effective COVID-19 therapeutics, especially in most low- and middle-income countries, the promising data on fluvoxamine, coupled with its low cost, known good tolerability [47], and wide availability, support the importance of prospective interventional studies on fluvoxamine for outpatient and inpatient therapy of COVID-19 [9–11].
To evaluate the efficacy and tolerability of fluvoxamine in terms of mortality among inpatients with COVID-19, we performed a prospective interventional open-label cohort study. This study was performed during the third wave of SARS-CoV-2 infections in Uganda, marked by the Omicron variant.
Materials and methods
Design and patients
We prospectively included all COVID-19 inpatients treated at the COVID-19 treatment unit (CTU) of Mulago Hospital in Uganda from December 2021 to February 2022. We collected data on clinical characteristics, comorbidities, coprescribed treatments, disease severity, and treatment outcomes (i.e., all-cause mortality, duration of hospitalization and complete symptom resolution). The details of the Mulago Hospital CTU have been previously described [48]. Briefly, to be admitted or treated for COVID-19, patients had to have either a positive SARS-CoV-2 reverse transcriptase-polymerase chain reaction (RT‒PCR) test or a positive COVID-19 rapid antigen SARS-CoV-2 test (COVID-19 RDT) or clinical signs and radiological tests consistent with a COVID-19 diagnosis. After evaluation for any specific medical contraindication or expected deleterious drug‒drug interaction [49], fluvoxamine was offered at the discretion of the physician at a dose of 100 mg twice a day for 10 days (based on the dose and duration used in the TOGETHER trial [18]), in addition to standard care, to all patients who consented to compassionate use. The exclusion criteria included age less than 18 years and unstable medical comorbidities as judged by the admitting physician, including severe underlying lung disease (i.e., chronic obstructive pulmonary disease (COPD), interstitial lung disease and pulmonary hypertension), decompensated cirrhosis, congestive heart failure (New York Heart Association (NYHA) stage C or D), solid organ transplant, psychiatric disorders with behavioral problems, or increased risk of bleeding. All patients were followed up during hospitalization until discharge or death, and no patients were lost to follow-up. All patients (taking or not taking fluvoxamine) were monitored by the treating physician for FDA-listed known [49] or potentially unknown side effects of fluvoxamine.
Patient management
Patients were treated according to national guidelines for the diagnosis and management of COVID-19 [50], as detailed in the Supplementary Text.
Main and secondary outcomes
The study baseline was defined as the date of hospital admission. The end of the study was death or discharge. The main outcome was all-cause mortality. Secondary outcomes were hospital discharge and complete symptom resolution, which was defined as having none of the COVID-19 symptoms that were present at admission, based on patient self-reports.
Statistical analysis
We used percentages for categorical variables and medians (interquartile ranges (IQRs)) for continuous variables to describe patients’ sociodemographic, COVID-19 symptom, vaccination status, comorbidity, and disease severity characteristics at admission. These characteristics were compared between patients taking versus those not taking fluvoxamine using standardized mean differences (SMDs) [51]. To examine the associations of fluvoxamine with the main outcome (all-cause mortality) and the two secondary outcomes (discharge and complete symptom resolution), we first performed univariate comparisons using SMDs for all outcomes as well as chi-squared tests (χ2) for binary outcomes and two-sample Mood’s median tests for continuous outcomes.
Next, we used time-to-event analyses adjusted for baseline sociodemographic and clinical characteristics and performed Cox proportional hazards regression models to examine the association of fluvoxamine with all-cause mortality and hospital discharge. To help account for the nonrandomized prescription of fluvoxamine and reduce the effects of confounding, the primary analyses used a propensity score analysis with inverse probability weighting (IPW) [52–55]. The individual propensities for exposure to fluvoxamine were estimated by multivariable logistic regression models that included all baseline characteristics (listed in Table 1 and in the footnotes of Table 2). In the IPW analyses, the predicted probabilities from the propensity score models were used to calculate the stabilized inverse probability weights [52, 53]. Associations of fluvoxamine with both outcomes were then estimated using IPW Cox regression models. In the case of nonbalanced covariates, IPW multivariable Cox regression models adjusting for the nonbalanced covariates were also performed. All Cox regression models estimating the association of fluvoxamine use with mortality were right-censored at the time of discharge. Kaplan‒Meier curves were drawn using the inverse probability weights [56], and their pointwise 95% confidence intervals were estimated using the nonparametric bootstrap method [57]. To examine the association of fluvoxamine with complete symptom resolution, we performed a multivariable logistic regression model that adjusted for all baseline characteristics and duration of follow-up and considered death as a failure to resolve symptoms. For all analyses, in the case of outliers, defined as values outside the 1.5 interquartile range (IQR), additional analyses excluding these outliers were also performed.
Table 1.
Clinical characteristics of participants treated and not treated with fluvoxamine (N = 316).
| Total | Fluvoxamine | No fluvoxamine | Fluvoxamine versus No fluvoxamine | ||||
|---|---|---|---|---|---|---|---|
| N = 316 | N = 94 | N = 222 | Full samplea | IPWb | |||
| N (%) | N (%) | N (%) | χ2 (p value)/Z (p value) | SMD | χ2 (p value)/Z (p value) | SMD | |
| Characteristics | |||||||
| Age (years)—Median (IQR) | 60.0 (37.0) | 60.0 (32.0) | 59.5 (39.5) | <0.01 (0.96) | 0.014 | 1.09 (0.28) | 0.062 |
| Sex | <0.01 (>0.99) | 0.003 | 0.01 (0.50) | 0.099 | |||
| Male | 151 (47.8) | 45 (47.9) | 106 (47.7) | ||||
| Female | 165 (52.2) | 49 (52.1) | 116 (52.3) | ||||
| COVID-19 symptoms | |||||||
| Any symptom | 297 (94.0) | 92 (97.9) | 205 (92.3) | 2.67 (0.10) | 0.258 | 2.78 (0.23) | 0.262 |
| Fever | 76 (24.1) | 30 (31.9) | 46 (20.7) | 3.94 (0.04) | 0.256 | 2.06 (0.18) | 0.068 |
| Cough | 253 (80.1) | 79 (84.0) | 174 (78.4) | 1.00 (0.32) | 0.145 | 2.32 (0.37) | 0.069 |
| Dyspnea | 173 (54.7) | 47 (50.0) | 126 (56.8) | 0.96 (0.33) | 0.136 | 1.67 (0.56) | 0.058 |
| Muscle ache | 26 (8.23) | 8 (8.51) | 18 (8.11) | <0.01 (>0.99) | 0.015 | 0.26 (0.13) | 0.085 |
| Delirium | 6 (1.90) | 1 (1.06) | 5 (2.25) | 0.07 (0.80) | 0.093 | 0.38 (0.23) | 0.019 |
| Headache | 50 (15.8) | 17 (18.1) | 33 (14.9) | 0.30 (0.58) | 0.087 | 0.80 (0.89) | 0.062 |
| Pharyngitis | 10 (3.16) | 1 (1.06) | 9 (4.05) | 1.07 (0.30) | 0.190 | 0.19 (0.77) | 0.027 |
| Rhinorrhea | 6 (1.90) | 1 (1.06) | 5 (2.25) | 0.07 (0.80) | 0.093 | 0.37 (0.21) | 0.065 |
| Chest pain | 84 (26.6) | 33 (35.1) | 51 (23.0) | 4.38 (0.04) | 0.270 | 1.26 (0.67) | 0.037 |
| Diarrhea | 7 (2.22) | 3 (3.19) | 4 (1.80) | 0.12 (0.73) | 0.089 | 1.26 (0.63) | 0.003 |
| Nausea or vomiting | 7 (2.22) | 1 (1.06) | 6 (2.70) | 0.24 (0.63) | 0.121 | 0.49 (0.11) | 0.063 |
| Vital signs | |||||||
| Temperature (°C)—Median (IQR)c | 37.2 (2.0) | 37.7 (1.78) | 37.1 (2.1) | 2.71 (0.10) | 0.168 | 0.48 (0.64) | 0.121 |
| Respiratory rate (breaths per minute)—Median (IQR) | 20.0 (32.5) | 19.0 (31.0) | 29.8 (33.0) | 0.38 (0.71) | 0.018 | 1.51 (0.13) | 0.175 |
| Pulse rate (bpm)—Median (IQR) | 92.0 (29.0) | 87.0 (23.8) | 96.0 (30.8) | 7.67 (<0.001) | 0.293 | 0.98 (0.32) | 0.026 |
| Blood pressure (mm Hg) | 0.25 (0.25) | 0.157 | 1.49 (0.29) | 0.005 | |||
| ≤130/90 | 218 (69.0) | 60 (63.8) | 158 (71.2) | ||||
| >130/90 | 98 (31.0) | 34 (36.2) | 64 (28.8) | ||||
| Comorbidities | |||||||
| Any comorbidity | 153 (48.4) | 46 (48.9) | 107 (48.2) | <0.01 (>0.99) | 0.015 | 0.88 (0.24) | 0.029 |
| Tuberculosis | 11 (3.48) | 0 (0.00) | 11 (4.95) | 3.46 (0.06) | 0.323 | 3.21 (0.001) | 0.227 |
| Heart disease (CVD) | 49 (15.5) | 17 (18.1) | 32 (14.4) | 0.43 (0.51) | 0.010 | 1.06 (0.81) | 0.030 |
| Asthma | 8 (2.53) | 3 (3.19) | 5 (2.25) | 0.01 (0.93) | 0.058 | 0.68 (0.48) | 0.021 |
| COPD | 3 (0.95) | 2 (2.13) | 1 (0.45) | 0.59 (0.44) | 0.149 | 3.60 (0.68) | 0.030 |
| Diabetes | 83 (26.3) | 25 (26.6) | 58 (26.1) | <0.01 (>0.99) | 0.011 | 0.01 (0.36) | 0.096 |
| Cancer | 8 (2.53) | 1 (1.06) | 7 (3.15) | 0.47 (0.49) | 0.146 | 1.19 (0.31) | 0.113 |
| HIV | 42 (13.3) | 9 (9.57) | 33 (14.9) | 1.18 (0.28) | 0.162 | 2.07 (0.35) | 0.015 |
| Oxygen therapy at admission | 14.76 (0.001) | 0.462 | 2.18 (0.72) | 0.032 | |||
| No supplemental O2 | 237 (75.0) | 57 (60.6) | 180 (81.1) | ||||
| <10 l/min | 50 (15.8) | 23 (24.5) | 27 (12.2) | ||||
| 10+ l/min | 29 (9.18) | 14 (14.9) | 15 (6.76) | ||||
| Vaccination status | 2.71 (0.26) | 0.193 | 3.13 (0.36) | 0.047 | |||
| At least 1 dose of COVID-19 vaccine | 81 (25.6) | 29 (30.9) | 52 (23.4) | ||||
| 1 dose | 33 (10.4) | 10 (10.6) | 23 (10.4) | ||||
| 2 doses | 48 (15.2) | 19 (20.2) | 29 (13.1) | ||||
| Not vaccinated | 235 (74.4) | 65 (69.1) | 170 (76.6) | ||||
| Co-prescribed COVID-19 medications | |||||||
| Dexamethasone | 232 (73.4) | 70 (74.5) | 162 (73.0) | 0.02 (0.89) | 0.034 | 0.05 (0.65) | 0.038 |
| Inhaled budesonide | 16 (5.06) | 7 (7.45) | 9 (4.05) | 0.95 (0.33) | 0.146 | 2.38 (0.69) | <0.001 |
| Antibiotics | 159 (50.3) | 48 (51.1) | 111 (50.0) | <0.01 (0.96) | 0.021 | 0.13 (0.16) | 0.120 |
| COVID-19 diagnostic methods | <0.01 (0.99) | 0.059 | 0.64 (0.62) | 0.017 | |||
| Positive RT-PCR | 5 (1.58) | 2 (2.13) | 3 (1.35) | ||||
| Positive RDT | 311 (98.4) | 92 (97.9) | 219 (98.6) | ||||
CVD cardiovascular disease, COPD chronic obstructive pulmonary disease, RDT antigen detection rapid diagnostic test, bpm beats per minute.
aWe used chi-squared tests (χ2) or Fisher’s exact tests if necessary for categorical variables and two-sample Mood’s median tests for continuous variables.
bWe used weighted chi-squared tests (χ2) for categorical variables and weighted two-sample Mood’s median tests for continuous variables.
cTemperature was measured using the axillary route.
Table 2.
Association of fluvoxamine with all-cause mortality and complete symptom resolution and, among survivors, with hospital discharge.
| Patients with fluvoxamine | Patients without fluvoxamine | Crude Cox regression analysis | Multivariable Cox regression analysisa | Cox regression analysis weighted by IPWb | Cox regression analysis weighted by IPW adjusted for unbalanced covariates | Cox regression analysis weighted by IPW adjusted for unbalanced covariates after excluding outliers | |
|---|---|---|---|---|---|---|---|
| Events/N (%) | Events/N (%) | HR (95% CI; p value) | AHR (95% CI; p value) | HR (95% CI; p value) | AHR (95% CI; p value) | AHR (95% CI; p value) [number of outliers] | |
| Mortality | |||||||
| Fluvoxamine | 29/94 (30.9) | 126/222 (56.8) | 0.44 (0.30–0.67; <0.001) | 0.29 (0.18–0.47; <0.001) | 0.33 (0.15–0.72; 0.005) | 0.32 (0.19–0.53; <0.001)c | 0.34 (0.23–0.51; <0.001) [9]f |
| Hospital discharge among survivors | |||||||
| Fluvoxamine | 65/65 (100) | 96/96 (100) | 0.86 (0.62–1.18; 0.34) | 0.89 (0.58–1.37; 0.60) | 0.81 (0.54–1.23; 0.32) | 0.86 (0.59–1.25; 0.43)d | 0.87 (0.60–1.27; 0.46) [8]g |
| Patients with fluvoxamine | Patients without fluvoxamine | Crude logistic regression analysis | Multivariable logistic regression analysisa | Multivariable logistic regression analysise | Multivariable logistic regression analysis after excluding outlierse | ||
| Events/N (%) | Events/N (%) | OR (95% CI; p value) | AOR (95% CI; p value) | AOR (95% CI; p value) | AOR (95% CI; p value) | ||
| Complete symptom resolution | |||||||
| Fluvoxamine | 51/94 (54.3) | 69/222 (31.1) | 2.63 (1.61–4.33; <0.001) | 2.56 (1.53–5.51; 0.001) | 2.13 (1.03–4.40; 0.04) | 3.30 (1.45–7.71; 0.005) [11]h | |
aAdjusted for age, sex, fever, cough, dyspnea, muscle ache, delirium, headache, pharyngitis, rhinorrhea, chest pain, diarrhea, and nausea or vomiting, temperature, respiratory rate, pulse rate, blood pressure, tuberculosis, heart disease, asthma, COPD, diabetes, cancer, HIV, oxygen therapy at admission, vaccination status, dexamethasone, inhaled budesonide, antibiotics, and method of COVID-19 diagnosis (degrees of freedom (df) = 31, all generalized variance inflation factors (GVIFs) <1.9).
bThe individual propensities for exposure to fluvoxamine were estimated by multivariable logistic regression models that included age, sex, fever, cough, dyspnea, muscle ache, delirium, headache, pharyngitis, rhinorrhea, chest pain, diarrhea, and nausea or vomiting, temperature, respiratory rate, pulse rate, blood pressure, tuberculosis, heart disease, asthma, COPD, diabetes, cancer, HIV, oxygen therapy at admission, vaccination status, dexamethasone, inhaled budesonide, antibiotics, and method of COVID-19 diagnosis (df = 31).
cAdjusted for temperature, respiratory rate, tuberculosis, cancer, and antibiotics.
dAdjusted for muscle ache, delirium, pharyngitis, pulse rate, tuberculosis, COPD, cancer, and HIV.
eAdjusted for age, sex, fever, cough, dyspnea, muscle ache, delirium, headache, pharyngitis, rhinorrhea, chest pain, diarrhea, and nausea or vomiting, temperature, respiratory rate, pulse rate, blood pressure, tuberculosis, heart disease, asthma, COPD, diabetes, cancer, HIV, oxygen therapy at admission, vaccination status, dexamethasone, inhaled budesonide, antibiotics, method of COVID-19 diagnosis, and duration of follow-up (df = 32, all GVIF < 1.8).
fFluvoxamine: Events/N (%) = 29/93 (31.2%); No fluvoxamine: Events/N (%) = 123/214 (57.5%).
gFluvoxamine: N = 61; No fluvoxamine: N = 92.
hFluvoxamine: Events/N (%) = 50/90 (55.6%); No fluvoxamine: Events/N (%) = 63/215 (29.3%).
We performed two sensitivity analyses. First, we used nearest neighbor matching to obtain a matched analytic sample [58] using a 1:1 ratio in the full sample and in the subsample of survivors, based on the same variables used for the IPW analysis. In these matched samples, we performed univariate Cox regression models for mortality and discharge and a univariate logistic regression model for complete symptom resolution. In the case of nonbalanced covariates, multivariable regression models adjusting for the nonbalanced covariates were also performed. Second, we reproduced the main analysis for mortality while censoring at day 15 and 28.
In the case of a significant association between fluvoxamine use and any outcome in the primary IPW analysis, we planned to calculate the between-group difference in absolute risk reduction/increase (ARR/ARI) [59] and number needed to treat (NNT) [59, 60] while taking into account the weighted time-to-event design when used and to perform post hoc exploratory analyses evaluating interactions of fluvoxamine with each baseline characteristic (e.g., vaccination status, baseline oxygen level) to assess their potential impact on the association between the outcome and fluvoxamine use.
The rates of adverse events of fluvoxamine [49] were also calculated and compared between inpatients with COVID-19 who did and did not receive the treatment.
For all associations, we performed residual analyses to assess the fit of the data, checked assumptions, including the proportional hazards assumption, using proportional hazards tests and diagnostics based on weighted residuals [61, 62], and examined the potential influence of outliers. We followed the recommendations of the STROBE reporting guideline for cohort studies. All analyses were performed using R software, version 4.2.1 [63]. Statistical significance was fixed a priori at a two-sided p value <0.05.
Results
Clinical characteristics
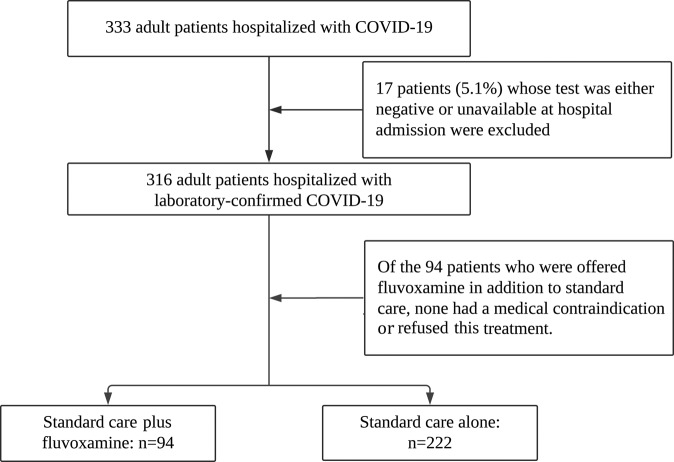
Of the 333 adult inpatients with COVID-19 treated at the CTU of Mulago Hospital, 17 (5.1%) patients were excluded because their COVID-19 biological test was either negative or unavailable at admission (Fig. 1). Of the remaining 316 patients, 94 (29.7%) were proposed fluvoxamine in addition to standard care, and all these patients accepted it. The median age of the patients was 60.0 years (IQR = 37.0), and 52.2% were women. The participants’ characteristics are summarized in Table 1. Compared with those without fluvoxamine, patients with fluvoxamine were more likely to have COVID-19 symptoms, COPD, oxygen therapy at admission, at least one dose of COVID-19 vaccine, coprescribed inhaled budesonide, higher temperature and blood pressure, and lower pulse rate; they were less likely to have tuberculosis, cancer, and HIV (Table 1). After applying the propensity score weights, these differences according to fluvoxamine exposure were reduced (Table 1). The distribution of characteristics in the subsample of survivors and in the matched analytic samples is given in eTables 1–3. The distribution of the inverse propensity score weights is given in eFigs. 1 and 2.
Fig. 1. Study cohort.
Study flow diagram showing participant recruitment.
Effectiveness of fluvoxamine
Over a median follow-up of 5 days (IQR = 5 days) until discharge or death, 155 patients (49.05%) died. A total of 29 patients (30.9%) who took fluvoxamine and 126 patients (56.8%) who did not died during hospitalization. Univariate comparison of mortality rates indicated a significantly lower mortality rate among patients who received fluvoxamine versus those who did not (SMD = 0.53; χ2 = 16.71, p < 0.001). Univariate comparison of complete symptom resolution indicated a significantly higher rate of symptom resolution among patients who received fluvoxamine versus those who did not [fluvoxamine group: 51/94 (54.3%); comparison group: 69/222 (31.1%); SMD = 0.49; χ2 = 14.09, p < 0.001]. Among the 161 survivors, univariate comparison of the median time from hospital admission to discharge did not show a significant difference [fluvoxamine group: median (IQR) = 7 (6); comparison group: median (IQR) = 6 (6); SMD = 0.27; two-sample Mood’s median test = 0.30, p = 0.76].
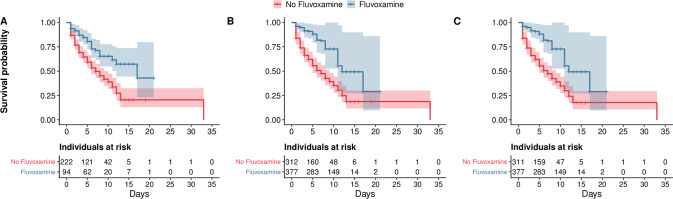
In the full sample of 316 inpatients, the unadjusted analysis (hazard ratio (HR) = 0.44; 95% CI = 0.30–0.67; p < 0.001), multivariable Cox regression analysis (AHR = 0.29; 95% CI = 0.18–0.47; p < 0.001), primary analysis with IPW (HR = 0.33; 95% CI = 0.15–0.72; p = 0.005), multivariable IPW Cox regression adjusting for unbalanced variables (AHR = 0.32; 95% CI = 0.19–0.53; p < 0.001), and multivariable IPW Cox regression excluding outliers (AHR = 0.34; 95% CI = 0.23–0.51; p < 0.001) showed a significant and substantial association between fluvoxamine use and reduced mortality (Table 2 and Fig. 2), corresponding to an ARR of death of 22.4% and an NNT of 4.46.
Fig. 2. Kaplan-Meier curves for all-cause mortality.
The shaded areas represent point wise 95% CI. A Kaplan-Meier curves for all-cause mortality in the crude analysis. B Kaplan-Meier curves for all-cause mortality in the analysis with inverse probability weighting (IPW). C Kaplan-Meier curves for all-cause mortality in the analysis with IPW while excluding outliers.
Fluvoxamine was significantly associated with greater rates of complete symptom resolution in the fully adjusted multivariable logistic regression model (AOR = 2.13; 95% CI = 1.03–4.40; p = 0.04), corresponding to an ARI of 23.2% and an NNT of 4.44, excluding outliers (AOR = 3.30; 95% CI = 1.45–7.71; p = 0.005).
Among survivors, fluvoxamine was not significantly associated with time to hospital discharge in the IPW Cox regression models (AHR = 0.86; 95% CI = 0.59–1.25; p = 0.43) or in the IPW Cox regression models that excluded outliers (AHR = 0.87; 95% CI = 0.60–1.27; p = 0.46) (Table 2 and eFig. 3).
In sensitivity analyses, the univariate Cox regression models in the 1:1 ratio matched analytic samples showed similar results (eTable 4 and eFig. 4), as did the primary analyses for mortality while censoring at day 15 and 28 (eTable 5).
Exploratory analyses suggested that the associations of fluvoxamine with mortality and complete symptom resolution did not significantly differ according to participants’ characteristics (eTables 6 and 7). In particular, the association of fluvoxamine with mortality was significant among both nonvaccinated patients and patients who had received at least 1 dose of the COVID-19 vaccine.
Tolerability of fluvoxamine
The frequency of side effects, mostly light or mild, was higher, albeit not significantly, among participants who received fluvoxamine (7.45%, N = 7) than among those who did not (3.15%, N = 7) (SMD = 0.21, χ2 = 3.46, p = 0.06) (Table 3).
Table 3.
Frequency of adverse events among participants treated and not treated with fluvoxamine (N = 316).
| Adverse events | Fluvoxamine (n = 94) | No fluvoxamine (n = 222) |
|---|---|---|
| N (%) | N (%) | |
| Nausea | 0 (0.0) | 1 (0.5) |
| Vomiting | 0 (0.0) | 1 (0.5) |
| Abdominal pain | 0 (0.0) | 1 (0.5) |
| Constipation | 0 (0.0) | 0 (0.0) |
| Diarrhea | 0 (0.0) | 0 (0.0) |
| Appetite loss | 3 (3.2) | 0 (0.0) |
| Xerostomia | 0 (0.0) | 0 (0.0) |
| Drowsiness | 0 (0.0) | 0 (0.0) |
| Insomnia | 0 (0.0) | 0 (0.0) |
| Dizziness | 0 (0.0) | 1 (0.5) |
| Nervousness | 0 (0.0) | 0 (0.0) |
| Anxiety | 0 (0.0) | 0 (0.0) |
| Headache | 3 (3.2) | 1 (0.5) |
| Muscle weakness | 0 (0.0) | 0 (0.0) |
| Paresthesia | 0 (0.0) | 0 (0.0) |
| Dysgeusia | 0 (0.0) | 0 (0.0) |
| Tachycardia | 0 (0.0) | 0 (0.0) |
| Hyperhidrosis | 0 (0.0) | 0 (0.0) |
| Syncope | 0 (0.0) | 0 (0.0) |
| Seizure | 0 (0.0) | 0 (0.0) |
| Glaucoma | 0 (0.0) | 0 (0.0) |
| Blurred vision | 0 (0.0) | 0 (0.0) |
| Back pain | 0 (0.0) | 1 (0.5) |
| Cough | 1 (1.1) | 0 (0.0) |
| Delirium | 0 (0.0) | 1 (0.5) |
| At least one of above | 7 (7.5) | 7 (3.2) |
Discussion
This prospective, interventional, open-label cohort study was a real-world evidence study supporting that 100 mg of fluvoxamine prescribed twice a day for 10 days in addition to standard care was significantly associated with reduced mortality and increased complete symptom resolution compared with standard care alone among adult patients hospitalized for COVID-19 in Uganda. These associations did not significantly differ according to clinical characteristics, including vaccination status. The frequency of side effects, mostly light or mild, was relatively low but higher, albeit not significantly, among participants who received fluvoxamine versus those who did not. Among survivors, no significant difference was found in terms of the duration of hospitalization.
These findings are in many ways consistent with prior preclinical and observational research findings suggesting that certain antidepressants, including fluvoxamine, could be beneficial against COVID-19 [9, 13, 17, 19, 23, 25, 33–36, 64, 65]. They are also consistent with the findings of three studies, including two RCTs and one nonrandomized open-label clinical study, which found a significant association between the short-term use (10–15 days) of fluvoxamine within 7 days of symptom onset and a reduced risk of clinical deterioration among outpatients with COVID-19 [17–19]. Specifically, in the STOP-COVID trial, clinical deterioration occurred in 0 of 80 patients in the fluvoxamine group and in 6 of 72 patients in the placebo group (absolute difference, 8.7% [95% CI, 1.8–16.4%] log-rank p = 0.009) [19]. The TOGETHER trial performed in an ambulatory setting reported a significantly lower risk of emergency department retention >6 h or hospital admission with fluvoxamine use compared with placebo [79 [11%] of 741 versus 119 [16%] of 756; relative risk [RR] 0.68; 95% Bayesian credible interval [95% BCI] = 0.52–0.88] [18, 66]. Three recent systematic reviews and meta-analyses reported that fluvoxamine significantly and substantially reduced hospitalization risk among outpatients with COVID-19 [13–16]. Finally, a prospective cohort study of patients admitted to the ICU for COVID-19 reported a significant association between the 15-day use of fluvoxamine and reduced mortality [20].
An RCT of fluvoxamine [21] prescribed at 100 mg/day among overweight and obese outpatients with COVID-19 showed no significant benefit on the risk of emergency department visits, hospitalizations or death, contrasting with the positive findings of TOGETHER and STOP-COVID, in which fluvoxamine was prescribed at doses of 200 and 300 mg/day, respectively. This discrepancy may be explained by a potential effect of fluvoxamine that occurs at a minimum dose of 200 mg/d, as suggested by two observational studies [36, 38] that found that exposure to antidepressants, especially those with FIASMA properties such as fluvoxamine, was associated with a reduced incidence of emergency department visits or hospital admissions among SARS-CoV-2-positive outpatients and with reduced 28-day mortality among COVID inpatients in a dose-dependent manner and from daily doses of at least 20 mg of fluoxetine equivalents. These findings suggest that fluvoxamine should be prescribed at a minimum daily dose of 200 mg (100 mg twice daily) to observe a benefit in patients with COVID-19, as was done in this study.
Overall, a relatively small proportion (7.45%) of patients who took fluvoxamine for 10 days reported side effects, mostly light or mild symptoms known to occur with fluvoxamine, and none of them were serious, with transient headache and appetite loss being the most common symptoms encountered, which is also consistent with COVID-19 symptoms. The frequency of side effects was nonetheless higher, albeit not significantly, in the fluvoxamine group.
The main strength of this study is that we provide for the first time, to our knowledge, real-world data on the efficacy and tolerability of fluvoxamine among inpatients with COVID-19 in Africa. However, this study has several limitations. First, because of the study design (nonrandomized and unblinded study), biases may have occurred at different levels. However, the use of mortality as the main outcome and the congruence of our observations with the findings of other studies, including a large randomized, placebo-controlled, prospective trial among high-risk outpatients [18], reduce this concern. Furthermore, we used different statistical approaches and sensitivity analyses that yielded similar results, suggesting the robustness of our results. Second, we did not collect data on viral clearance or inflammatory markers, preventing us from assessing the mechanisms through which fluvoxamine confers benefits against COVID-19. Third, the use of the exclusion criterion “unstable medical comorbidities” as judged by the admitting physician instead of a more objective measure might have introduced a potential bias. Fourth, data on potential deaths after hospital discharge were not available. Finally, we were not able to determine treatment adherence or potential unexpected drug‒drug interactions through the blood dosage of fluvoxamine.
In conclusion, this study confirms and extends prior RCT results in finding that fluvoxamine prescribed at 100 mg twice a day is well tolerated and significantly associated with reduced mortality and increased complete symptom resolution among COVID-19 inpatients. RCTs of fluvoxamine prescribed at a daily dose of 200 mg are urgently needed to confirm these results among inpatients with COVID-19.
Supplementary information
Acknowledgements
We acknowledge the leadership of the Mulago National Referral Hospital for the administrative support offered during this study. We appreciate the contribution of health workers at the CTU, medical officers, nurses and research assistants who collected data. We also acknowledge Julien Potet of the Médecins Sans Frontières Access Campaign, Paris, France for the input to the manuscript. We acknowledge the funding support from the Government of the Republic of Uganda through the Makerere University Research and Innovations Fund (Mak-RIF).
Author contributions
Concept and design: BJK; Funding acquisition: BJK and WB; Acquisition of data: RM, EM, EK, VN, LON, RS, WK and HA; Administrative, technical, or material support: WM, IS, HK, RB and HGM; Supervision: BJK and WM; Critical revision of the manuscript: BJK, LM, MSR, NH, WM, PBK and WB; Statistical analysis: BJK, LM, MSR and NH.
Code availability
All data and the R code used for this study are available at https://github.com/mlsrico/fluvoxamine_uganda.
Competing interests
NH is an inventor on a patent application related to methods of treating COVID-19 (FIASMA antidepressants), filled by Assistance Publique-Hopitaux de Paris in France. The other authors have no conflicts of interest related to this work to declare.
Ethical approval
Compassionate use approval of fluvoxamine for the treatment of COVID-19 was obtained from the Ministry of Health of Uganda (clearance reference no. ADM.180/O1 dated 2nd January 2022). All patients were informed that this medication was under evaluation by health authorities. Patients willing to be treated with this medication gave verbal informed consent. All data used in this study were collected as part of routine care.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-023-02004-3.
References
- 1.Ciotti M, Ciccozzi M, Terrinoni A, Jiang WC, Wang CB, Bernardini S. The COVID-19 pandemic. Crit Rev Clin Lab Sci. 2020;57:365–88. doi: 10.1080/10408363.2020.1783198. [DOI] [PubMed] [Google Scholar]
- 2.Chevance A, Gourion D, Hoertel N, Llorca PM, Thomas P, Bocher P, et al. Ensuring mental health care during the SARS-CoV-2 epidemic in France: a narrative review. L’Encéphale. 2020;46:193–201. doi: 10.1016/j.encep.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoertel N, Blachier M, Blanco C, Olfson M, Masseti M, Sánchez-Rico M, et al. A stochastic agent-based model of the SARS-CoV-2 epidemic in France. Nat Med. 2020;26:1417–21. doi: 10.1038/s41591-020-1001-6. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Paulson KR, Pease SA, Watson S, Comfort H, Zheng P, et al. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet. 2022;399:1513–36. doi: 10.1016/S0140-6736(21)02796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musoke P, Okot J, Nanfuka V, Rwamafa P, Masajjage J, Kisuule I, et al. A preliminary report on herbal medicine use among patients hospitalized at two-large COVID-19 treatment centers in Uganda. RMHP. 2021;14:4609–17. doi: 10.2147/RMHP.S339408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keehner J, Horton LE, Pfeffer MA, Longhurst CA, Schooley R, Currier JS, et al. SARS-CoV-2 infection after vaccination in health care workers in California. N Engl J Med. 2021;384:1774–5. doi: 10.1056/NEJMc2101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim S, Tignanelli CJ, Hoertel N, Boulware DR, Usher MG. Prevalence of medical contraindications to nirmatrelvir/ritonavir in a cohort of hospitalized and nonhospitalized patients with COVID-19. Open Forum Infect Dis. 2022;9:ofac389. doi: 10.1093/ofid/ofac389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoertel N, Boulware DR, Sánchez-Rico M, Burgun A, Limosin F. Prevalence of contraindications to nirmatrelvir-ritonavir among hospitalized patients with COVID-19 at risk for progression to severe disease. JAMA Netw Open. 2022;5:e2242140. doi: 10.1001/jamanetworkopen.2022.42140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoertel N. Do the selective serotonin reuptake inhibitor antidepressants fluoxetine and fluvoxamine reduce mortality among patients with COVID-19. JAMA Netw Open. 2021;4:e2136510. doi: 10.1001/jamanetworkopen.2021.36510. [DOI] [PubMed] [Google Scholar]
- 10.Hoertel N, Sánchez-Rico M, Herrera-Morueco JJ, de la Muela P, Gulbins E, Kornhuber J, et al. Comorbid medical conditions are a key factor to understand the relationship between psychiatric disorders and COVID-19-related mortality: results from 49,089 COVID-19 inpatients. Mol Psychiatry. 2022;27:1278–80. doi: 10.1038/s41380-021-01393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoertel N, Sánchez-Rico M, Cougoule C, Gulbins E, Kornhuber J, Carpinteiro A, et al. Repurposing antidepressants inhibiting the sphingomyelinase acid/ceramide system against COVID-19: current evidence and potential mechanisms. Mol Psychiatry. 2021;26:7098–9. doi: 10.1038/s41380-021-01254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer KJ, Benfield P. Fluvoxamine: an overview of its pharmacological properties and review of its therapeutic potential in non-depressive disorders. CNS Drugs. 1994;1:57–87. [Google Scholar]
- 13.Lee TC, Vigod S, Bortolussi-Courval É, Hanula R, Boulware DR, Lenze EJ, et al. Fluvoxamine for outpatient management of COVID-19 to prevent hospitalization: a systematic review and meta-analysis. JAMA Netw Open. 2022;5:e226269. doi: 10.1001/jamanetworkopen.2022.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen W, Chen C, Tang J, Wang C, Zhou M, Cheng Y, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med. 2022;54:516–23. doi: 10.1080/07853890.2022.2034936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcec R, Dodig VM, Likic R. A meta-analysis regarding fluvoxamine and hospitalization risk of COVID-19 patients: TOGETHER making a difference. J Infect. 2022;S0163-4453:00672–7. doi: 10.1016/j.jinf.2022.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng J, Rayner D, Ramaraju HB, Abbas U, García C, Heybati K, et al. Efficacy and safety of selective serotonin reuptake inhibitors in COVID-19 management: a systematic review and meta-analysis. Clin Microbiol Infect. 2023;S1198-743X:00032–0. doi: 10.1016/j.cmi.2023.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seftel D, Boulware DR. Prospective cohort of fluvoxamine for early treatment of Coronavirus Disease 19. Open Forum Infect Dis. 2021;8:ofab050. doi: 10.1093/ofid/ofab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reis G, dos Santos Moreira-Silva EA, Silva DCM, Thabane L, Milagres AC, dos Santos CVQ, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2021:S2214109X21004484. 10.1016/S2214-109X(21)00448-4. [DOI] [PMC free article] [PubMed]
- 19.Lenze EJ, Mattar C, Zorumski CF, Stevens A, Schweiger J, Nicol GE, et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324:2292–300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calusic M, Marcec R, Luksa L, Jurkovic I, Novac N, Mihaljevic S, et al. Safety and efficacy of fluvoxamine in COVID-19 ICU patients: an open label, prospective cohort trial with matched controls. Br J Clin Pharmacol. 2021. 10.1111/bcp.15126. [DOI] [PMC free article] [PubMed]
- 21.Bramante CT, Huling JD, Tignanelli CJ, Buse JB, Liebovitz DM, Nicklas JM, et al. Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19. N Engl J Med. 2022;387:599–610. doi: 10.1056/NEJMoa2201662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schloer S, Brunotte L, Goretzko J, Mecate-Zambrano A, Korthals N, Gerke V, et al. Targeting the endolysosomal host-SARS-CoV-2 interface by clinically licensed functional inhibitors of acid sphingomyelinase (FIASMA) including the antidepressant fluoxetine. Emerg Microbes Infect. 2020;9:2245–55. doi: 10.1080/22221751.2020.1829082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpinteiro A, Edwards MJ, Hoffmann M, Kochs G, Gripp B, Weigang S, et al. Pharmacological inhibition of acid sphingomyelinase prevents uptake of SARS-CoV-2 by epithelial cells. Cell Rep Med. 2020;1:100142. doi: 10.1016/j.xcrm.2020.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosen DA, Seki SM, Fernández-Castañeda A, Beiter R, Eccles J, Woodfolk JA, et al. Modulation of the sigma-1 receptor–IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci Transl Med. 2019;11:eaau5266. doi: 10.1126/scitranslmed.aau5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sukhatme VP, Reiersen AM, Vayttaden SJ, Sukhatme VV. Fluvoxamine: a review of its mechanism of action and its role in COVID-19. Front Pharm. 2021;12:652688. doi: 10.3389/fphar.2021.652688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto Y, Suzuki T, Hashimoto K. Mechanisms of action of fluvoxamine for COVID-19: a historical review. Mol Psychiatry. 2022;27:1898–907. doi: 10.1038/s41380-021-01432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kornhuber J, Hoertel N, Gulbins E. The acid sphingomyelinase/ceramide system in COVID-19. Mol Psychiatry. 2022;27:307–14. doi: 10.1038/s41380-021-01309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Court SDM. The definition of acute respiratory illnesses in children. Postgrad Med J. 1973;49:771–6. doi: 10.1136/pgmj.49.577.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt HR, Kruse AC. The molecular function of σ receptors: past, present, and future. Trends Pharmacol Sci. 2019;40:636–54. doi: 10.1016/j.tips.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishima T, Fujita Y, Hashimoto K. Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells. Eur J Pharmacol. 2014;727:167–73. doi: 10.1016/j.ejphar.2014.01.064. [DOI] [PubMed] [Google Scholar]
- 31.Gulbins E, Palmada M, Reichel M, Lüth A, Böhmer C, Amato D, et al. Acid sphingomyelinase–ceramide system mediates effects of antidepressant drugs. Nat Med. 2013;19:934–8. doi: 10.1038/nm.3214. [DOI] [PubMed] [Google Scholar]
- 32.Carpinteiro A, Gripp B, Hoffmann M, Pöhlmann S, Hoertel N, Edwards MJ, et al. Inhibition of acid sphingomyelinase by ambroxol prevents SARS-CoV-2 entry into epithelial cells. J Biol Chem. 2021;296:100701. doi: 10.1016/j.jbc.2021.100701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoertel N, Sánchez-Rico M, Gulbins E, Kornhuber J, Carpinteiro A, Lenze EJ, et al. Association between FIASMAs and reduced risk of intubation or death in individuals hospitalized for severe COVID-19: an observational multicenter study. Clin Pharm Ther. 2021;110:1498–511. doi: 10.1002/cpt.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoertel N, Sánchez-Rico M, Vernet R, Beeker N, Jannot AS, Neuraz A, et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. Mol Psychiatry. 2021;26:5199–212. doi: 10.1038/s41380-021-01021-4. [DOI] [PubMed] [Google Scholar]
- 35.Hoertel N, Sánchez-Rico M, Gulbins E, Kornhuber J, Carpinteiro A, Abellán M, et al. Association between FIASMA psychotropic medications and reduced risk of intubation or death in individuals with psychiatric disorders hospitalized for severe COVID-19: an observational multicenter study. Transl Psychiatry. 2022;12:90. doi: 10.1038/s41398-022-01804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoertel N, Sánchez-Rico M, Kornhuber J, Gulbins E, Reiersen AM, Lenze E, et al. Antidepressant use and its association with 28-day mortality in inpatients with SARS-CoV-2: support for the FIASMA model against COVID-19. JCM. 2022;11:5882. doi: 10.3390/jcm11195882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oskotsky T, Maric I, Tang A, Oskotsky B, Wong RJ, Aghaeepour N, et al. Mortality risk among patients with COVID-19 prescribed selective serotonin reuptake inhibitor antidepressants. JAMA Netw Open. 2021;4:e2133090. doi: 10.1001/jamanetworkopen.2021.33090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fritz BA, Hoertel N, Lenze EJ, Jalali F, Reiersen AM. Association between antidepressant use and ED or hospital visits in outpatients with SARS-CoV-2. Transl Psychiatry. 2022;12:341. doi: 10.1038/s41398-022-02109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fred SM, Kuivanen S, Ugurlu H, Casarotto PC, Levanov L, Saksela K, et al. Antidepressant and antipsychotic drugs reduce viral infection by SARS-CoV-2 and fluoxetine shows antiviral activity against the novel variants in vitro. Front Pharm. 2022;12:755600. doi: 10.3389/fphar.2021.755600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Wu Y, Chen S, Zhan Q, Wu D, Yang C, et al. Sertraline is an effective SARS-CoV-2 entry inhibitor targeting the spike protein. J Virol. 2022;96:e0124522. doi: 10.1128/jvi.01245-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khater SE, El-khouly A, Abdel-Bar HM, Al-mahallawi AM, Ghorab DM. Fluoxetine hydrochloride loaded lipid polymer hybrid nanoparticles showed possible efficiency against SARS-CoV-2 infection. Int J Pharm. 2021;607:121023. doi: 10.1016/j.ijpharm.2021.121023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunotte L, Zheng S, Mecate-Zambrano A, Tang J, Ludwig S, Rescher U, et al. Combination therapy with fluoxetine and the nucleoside analog GS-441524 exerts synergistic antiviral effects against different SARS-CoV-2 variants in vitro. Pharmaceutics. 2021;13:1400. doi: 10.3390/pharmaceutics13091400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dechaumes A, Nekoua MP, Belouzard S, Sane F, Engelmann I, Dubuisson J, et al. Fluoxetine can inhibit SARS-CoV-2 in vitro. Microorganisms. 2021;9:339. doi: 10.3390/microorganisms9020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schloer S, Brunotte L, Mecate-Zambrano A, Zheng S, Tang J, Ludwig S, et al. Drug synergy of combinatory treatment with remdesivir and the repurposed drugs fluoxetine and itraconazole effectively impairs SARS-CoV-2 infection in vitro. Br J Pharm. 2021;178:2339–50. doi: 10.1111/bph.15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimniak M, Kirschner L, Hilpert H, Geiger N, Danov O, Oberwinkler H, et al. The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2 in human lung tissue. Sci Rep. 2021;11:5890. doi: 10.1038/s41598-021-85049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Péricat D, Leon-Icaza SA, Sánchez-Rico M, Mühle C, Zoicas I, Schumacher F, et al. Antiviral and anti-inflammatory activities of fluoxetine in a SARS-CoV-2 infection mouse model. Int J Mol Sci. 2022;23:13623. doi: 10.3390/ijms232113623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Ogawa Y, Leucht S, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–66. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirenga B, Muttamba W, Kayongo A, Nsereko C, Siddharthan T, Luisba J, et al. Characteristics and outcomes of admitted patients infected with SARS-CoV-2 in Uganda. BMJ Open Resp Res. 2020;7:e000646. doi: 10.1136/bmjresp-2020-000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jazz Pharmaceuticals. Luvox (fluvoxamine maleate)—full prescribing information. 2008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/022235lbl.pdf.
- 50.Uyeki TM. Influenza. Ann Intern Med. 2021;174:ITC161–76. doi: 10.7326/AITC202111160. [DOI] [PubMed] [Google Scholar]
- 51.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–34. [Google Scholar]
- 52.Robins JM, Hernán MÁ, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 53.Hoertel N, Sánchez-Rico M, Gulbins E, Kornhuber J, Vernet R, Beeker N, et al. Association between benzodiazepine receptor agonist use and mortality in patients hospitalised for COVID-19: a multicentre observational study. Epidemiol Psychiatr Sci. 2022;31:e18. doi: 10.1017/S2045796021000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoertel N, Sánchez-Rico M, Vernet R, Jannot AS, Neuraz A, Blanco C, et al. Observational study of chlorpromazine in hospitalized patients with COVID-19. Clin Drug Investig. 2021;41:221–33. doi: 10.1007/s40261-021-01001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoertel N, Sánchez-Rico M, Vernet R, Jannot AS, Neuraz A, Blanco C, et al. Observational study of haloperidol in hospitalized patients with COVID-19. PLoS ONE. 2021;16:e0247122. doi: 10.1371/journal.pone.0247122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Efron B. Nonparametric standard errors and confidence intervals. Can J Stat. 1981;9:139–58. [Google Scholar]
- 57.Kassambara A, Kosinski M, Biecek P. Survminer: Drawing Survival Curves Using “Ggplot2”. 2020. https://CRAN.R-project.org/package=survminer.
- 58.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42. 10.18637/jss.v042.i08.
- 59.Ranganathan P, Pramesh C, Aggarwal R. Common pitfalls in statistical analysis: absolute risk reduction, relative risk reduction, and number needed to treat. Perspect Clin Res. 2016;7:51. doi: 10.4103/2229-3485.173773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Z. Nnt: the number needed to treat (NNT) for survival endpoint. 2020. https://CRAN.R-project.org/package=nnt.
- 61.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. Springer; New York, USA; 2000.
- 62.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 63.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Auckland, New Zealand; 2022. https://www.R-project.org/.
- 64.Clelland CL, Ramiah K, Steinberg L, Clelland JD. Analysis of the impact of antidepressants and other medications on COVID-19 infection risk in a chronic psychiatric in-patient cohort. BJPsych Open. 2021;8:e6. doi: 10.1192/bjo.2021.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Creeden JF, Imami AS, Eby HM, Gillman C, Becker KN, Reigle J, et al. Fluoxetine as an anti-inflammatory therapy in SARS-CoV-2 infection. Biomed Pharmacother. 2021;138:111437. doi: 10.1016/j.biopha.2021.111437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reis G, Mills E. Fluvoxamine for the treatment of COVID-19—author’s reply. Lancet Glob Health. 2022;10:e333. doi: 10.1016/S2214-109X(21)00588-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and the R code used for this study are available at https://github.com/mlsrico/fluvoxamine_uganda.