Abstract
Objective.
The purpose of this research was to investigate the correlation between serum levels of surfactant protein-D (SP-D) with acute respiratory distress syndrome (ARDS) severity and mortality in COVID-19.
Materials and Method.
This was a prospective cohort research study that included 76 patients in the period from July to October 2020. SP-D serum levels were taken upon admission to the hospital, the diagnosis of ARDS and its grade were confirmed according to the WHO criteria, and then patients were observed for 28-day mortality.
Results.
The mean SP-D serum levels from 76 patients were 39.33 ng/ml (SD±31.884 ng/ml). The statistical analysis showed that there was a significant correlation between SP-D serum levels and the severity of ARDS upon admission to the hospital (P=0.04, Spearman’s rank correlation coefficient (rs)=0.26), but the correlation between serum levels of SP-D and mortality was not statistically significant (P=0.89; rs=-0.016).
Conclusion.
SP-D serum levels had a significant but weak correlation with ARDS severity, but were not significant for mortality.
Keywords: Surfactant Protein-D, ARDS, Severity, Mortality, COVID-19
Introduction
In late 2019, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), caused an epidemic of an acute respiratory disease in Wuhan, China. WHO later referred to this disease as Coronavirus Disease 2019 (COVID-19) (1), and as of September 2021 the number of people diagnosed with COVID-19 worldwide surpassed 221 million. Acute Respiratory Distress Syndrome (ARDS) is the most common complication of COVID-19 (60-70% of patients admitted to the ICU), followed by shock (30%), myocardial dysfunction (20-30%) and acute kidney injury (10-30%) (2). An initial study in China reported that ARDS in COVID-19 had a 28-day mortality rate of 74% (3).
The severity of the lung injury often requires mechanical ventilation, and recently some morphological pathways of COVID-19-related ARDS have been elucidated in a series of autopsies. Histological analysis of pulmonary vessels showed widespread thrombosis with microangiopathy, diffuse alveolar damage, capillary congestion, necrosis of pneumocytes, interstitial and intra-alveolar edema, and platelet–fibrin thrombi. These results suggest that the profound hypoxemia that these patients might experience can be due to both epithelial and endothelial injury (4). A study by Sega et al. showed that endothelial markers (especially Endoglin and VCAM-1) are associated with mortality in COVID-19 cases (5).
Surfactant Protein-D (SP-D) is a biomarker of lung epithelial injury, which is produced by type-II alveolar cells and plays an important role in maintaining the integrity of the alveolar-capillary interface. The mechanism of SP-D in the alveolar entering the circulation system is not fully understood, but Hart et al. suggested that in inflammatory conditions, such as ARDS, increased alveolar-capillary permeability may lead to leakage of alveolar SP-D into the systemic circulation (6). SP-D is also thought to be a more specific marker for lung damage, so it can be used as a marker of early lung injury.
Several studies on SP-D have been conducted. One of them is a 2009 study by Delgado et al. that aimed to determine the correlation between SP-D and mortality in H1N1 virus infection. The results showed that higher SP-D serum levels were associated with the risk of death in patients with H1N1 pneumonia (7). Moreover, a recent study by Kerget et al. and Saito et al. examined SP-D in COVID-19 patients. These studies compared SP-D serum levels in a group of COVID-19 patients who had ARDS, and those who did not. SP-D, as a pulmonary epithelial maker, has been shown to be a prognostic biomarker that can predict the outcome of H1N1 virus infection and in non-COVID-19 cases, but there are not many studies about the role of SP-D in COVID-19.
The purpose of this study was to analyze the correlation between SP-D serum levels with ARDS severity and mortality in COVID-19 cases.
Method
This was a prospective cohort research study that included 76 patients. The study was performed in the COVID-19 isolation ward and ICU at Dr Soetomo Surabaya Hospital, in the period from July to October 2020, and was approved by the Ethics Comittee of Dr Soetomo Surabaya Hospital.
The study population consisted of patients treated in isolation wards and ICU at Dr Soetomo Surabaya Hospital in the period from July 2020 - October 2020. All patients who met the inclusion, and exclusion, criteria were included in the study sample until the minimum number of samples was met. The inclusion criteria were: confirmed diagnosis of COVID-19 and aged 18 years old or above. The exclusion criteria were: patients with COPD, asthma and tuberculosis infection; patients with an autoimmune disease, immunocompromised or those taking immunosuppressant drugs; and patients with malignancy or receiving chemotherapy treatment. The correlation sample size formula was used to calculate the minimum number of patients (α: 0.05; β: 0.2 and r: 0.5), and the minimum sample size was 30 patients.
The patients underwent a COVID-19 test, blood gas analysis and SP-D serum levels upon hospital admission. Diagnosis of COVID-19 was confirmed by an RT-PCR test from the patients’ nasopharyngeal swab samples; the PaO2/FiO2 ratio was used to determine ARDS severity; and the ELISA method (Human SP-D ELISA Kit by Elabscience®) was used to determine SP-D serum levels. The definition of ARDS and its severity were categorized using the WHO criteria: a PaO2/FiO2 ratio of <100 as severe ARDS, 100-200 as moderate ARDS, 200-300 as mild ARDS and >300 was categorized as not ARDS (6). The diagnosis of ARDS was made upon hospital admission by history taking, physical examination and laboratory tests. All the patients received Dr Soetomo Surabaya Hospital standard protocol therapy for COVID-19 that follows the WHO’s guidelines for Clinical Management of COVID-19.
Statistical Analysis
The IBM SPSS Statistics 23.0® program was used for statistical analysis. Continuous data were reported as means. Categorical data were presented as a percentage. The correlation between SP-D serum levels and ARDS severity and mortality were analyzed using the Spearman correlation test. A P value <0.05 was defined as statistically significant.
Results
Characteristics of Research Subjects
From 76 patients studied, 68 patients (89.5%) had comorbidities and 8 patients (10.5%) had no comorbidities. The most common comorbidities were obesity (64.7%), diabetes mellitus (48.5%) and hypertension (30.9%).The 51-60 years age group had the most patients who died (35.9%). However, when viewed on the basis of the proportion of the number of patients who survived and died in each age group, the >71 years age group was the worst, with a 100% mortality rate.
SP-D Serum Levels, ARDS Severity and Mortality
In this study, an analysis was also carried out to see the differences in the value of serum SP-D levels in patients who died and those who survived for up to 28 days of observation. The results obtained were the ARDS severity upon hospital admission (Table 2) and mortality after 28 days of observation (Table 3).
Table 2.
Severity of ARDS
| Severity | N (%) | PaO2/FiO2 ratio Mean±SD | SP-D (ng/mL) Mean±SD |
|---|---|---|---|
| Non ARDS | 11 (14.5) | 369.14±41.57 | 37.46±32.95 |
|
| |||
| Mild ARDS | 14 (18.4) | 230.81±23.46 | 29.87±26.63 |
|
| |||
| Moderate ARDS | 29 (38.2) | 145.03±30.10 | 32.67±30.35 |
|
| |||
| Severe ARDS | 22 (28.9) | 81.30±12.29 | 55.04±32.62 |
PaO2=Partial Oxygen Pressure in artery; FiO2 Fraction of Inspired Oxygen; SD=Standard Deviation; SP-D=Surfactant Protein-D; ARDS=Acute Respiratory Distress Syndrome.
Table 3.
Mortality of Research Subjects
| Outcome | N (%) | SP-D (ng/mL) Mean ± SD |
|---|---|---|
| Survived | 37 (48.7) | 38.92±32.00 |
|
| ||
| Died | 39 (51.3) | 39.70±32.18 |
|
| ||
| Died <14 days | 30 (39.5) | 37.74±31.69 |
|
| ||
| Died >14 days | 9 (11.8) | 46.22±34.87 |
SD=Standard Deviation; SP-D=Surfactant Protein-D.
Table 1.
Characteristics of Subjects
| Variables | Survivor (N=39) | Non Survivor (N=37) | P value |
|---|---|---|---|
| Age (years) Mean±SD | 55.56±12.96* | 49.43±10.29* | 0.026 |
|
| |||
| Gender | |||
|
| |||
| Male N (%) | 25 (64.1) | 21 (56.8) | 0.513 |
|
| |||
| Female N (%) | 14 (35.9) | 16 (43.2) | |
|
| |||
| BMI (kg/m2) | 26.67 (22.89-29,38)† | 26.04 (22.72-28.01)† | 0.323 |
|
| |||
| SP-D (ng/mL) | 24.48 (15.0-75.18) † | 24.07 (13.40-63.58)† | 0.775 |
|
| |||
| PaO2/FiO2 ratio | 117.8 (85.0-151.40)† | 206.7 (121.75-300.35)† | <0.001 |
|
| |||
| LOS (days) | 7 (4-13) | 22 (15-26.5) | <0.001 |
Data are reported as number (percentage);
Mean±standard deviation; Median [interquartile range] as appropriate. SD=Standard Deviation; BMI=Body Mass Index; SP-D=Surfactant Protein-D; PaO2=Partial Oxygen Pressure in artery; FiO2=Fraction of Inspired Oxygen; LOS=Length of Stays.
Correlation between SP-D Serum Levels and ARDS Severity in COVID-19 Patients
Statistical analysis was performed to determine the significance of the correlation between SP-D serum levels and the severity of ARDS in this study. The results of the Spearman correlation analysis showed a correlation coefficient (rs) of 0.236 with a P value=0.04. This means that there was a significant correlation between SP-D serum levels and the severity of ARDS upon hospital admission (P<0.05), with a weak correlation strength (rs: 0.20 - 0.39).
Correlation between SP-D Serum Levels with Mortality in COVID-19 Patients
Statistical analysis was calculated to see the significance of SP-D serum levels with mortality in this study. The results of the Spearman correlation analysis showed a correlation coefficient (rs) of -0.016 with P value = 0.89. This means that the correlation between SP-D serum levels upon hospital admission and mortality was not significant (P>0.05), with a very weak negative correlation strength (rs: 0.00 to -0.19).
Discussion
This study highlights that SP-D had a connection with ARDS severity in COVID-19, thus supporting the opinion that treatment using surfactants can provide benefits in COVID-19 cases. The purpose of our study was to give us a better understanding of the role of SP-D serum levels in COVID-19. We use 3 variables in this study: SP-D serum levels upon hospital admission; ARDS severity upon hospital admission; and mortality after 28 days of observation. The results of this study concluded that the correlation between SP-D serum levels upon hospital admission with ARDS severity upon hospital admission was statistically significant, with a weak correlation strength, but the correlation between SP-D serum levels with mortality was not statistically significant, with a very weak negative correlation strength.
Characteristics of the Research Subjects
The gender characteristics of this study was that it was dominated by male patients. The characteristics of these patients are similar to the study conducted by Zhi et al. where 63.8% of the 1023 confirmed COVID-19 patients studied were male. Male patients also dominated in the non-survivor group,which is similar to the systematic review conducted by Yustinawati et al. It was found that of a total of 1,314 COVID-19 patients who died, 845 patients (64%) were male (8). Gender is a risk factor for severe COVID-19. Sexual hormone-mediated immune responses and differences in ACE2 expression in the different sexes are thought to play a role in determining disease severity. It was explained that the females had a more effective adaptive immune response than the males. This may be due to the production of sex hormones and the difference in the number of genes related to immunity that are found more on the X chromosome (9, 10).
Age is a significant risk factor for COVID-19 because it is also associated with comorbidities and a decrease in the effectiveness of the immune system due to the physiological process of aging. A meta-analysis conducted by Booth et al. found that patients >75 years of age had a higher risk of contracting COVID-19 (11). A retrospective multicenter cohort study by Luo H et al., found that of 625 COVID-19 patients, there were 41.8% young adults (19 – 44 years old), 39.7% middle-aged adults (45 - 64 years old) and 12.6% elderly (≥ 65 years). In our study, the age of the patients was dominated by the 51 – 60 year age group and 41 – 50 years, which has similarities with the study by Luo et al (10). Elderly patients (≥ 65 years) with COVID-19 have the highest risk for severe or critical illness, intensive care, respiratory failure and length of hospital stay; which may be due to the higher incidence of comorbidities and decreased immunity to COVID-19 (12). Aging has been associated with modifications in signaling mechanisms responsible for IFN production, leading to reduced IFN production. Coronavirus, and specifically SARS-COV-2 infections, have been shown to induce IFN production poorly, a mechanism of possible viral immune escape. These immunological deficiencies can synergistically cooperate in a single patient leading to impaired IFN production, insufficient immune responses, and more severe manifestation of COVID-19 (13).
Obesity, diabetes mellitus and hypertension were the most common comorbidities suffered by the patients in this study. There is a clear correlation between obesity and basal inflammatory status, which is characterized by higher levels of IL-6 and CRP. Adipose tissue in obesity is “pro-inflammatory” and thus leads to increased expression of cytokines, especially adipokines. In the study by Roncon et al., it was reported that patients with diabetes mellitus had a nearly three times higher risk of admission to the ICU and death (14). A study by Chang et al. found from a multivariate logistic analysis that diabetes mellitus was significantly associated with disease progression of COVID-19. Diabetes is related to the progression of COVID-19 because hyperglycemia causes immune dysfunction, impaired neutrophil function, antioxidant system and humoral immunity, and causes a tendency to nosocomial infections that aggravate COVID-19 symptoms (15).Therapy for hypertension, diabetes mellitus and cardiovascular disease is needed to increase the expression of the ACE2 protein. In COVID-19 patients, SARS-CoV-2 binds to the ACE2 receptor, spreads and causes tissue damage, especially in organs with high ACE2 receptor expression(16). This causes an increase in viral load which eventually aggravates the disease and then triggers ARDS, cytokine storm and even death (7).
SP-D Serum Levels in COVID-19 Patients
On the basis of the central role of SP-D in lung defense, regulation of the inflammatory response and its dysregulation in lung disease, it was hypothesized that increased levels of SP-D in blood serum are caused by lung tissue damage (7). The mechanism by which SP-D in the alveolar can enter the circulation system is not fully understood, but Hart et al. suggested that in inflammatory conditions, such as ARDS, increased alveolar-capillary permeability may lead to leakage of alveolar SP-D into the systemic circulation. The integrity of the secretory epithelial cells can be damaged in lung inflammation, resulting in leakage of SP-D from the epithelial cells into the alveoli and then into the blood vessels.
In this study, the overall mean SP-D serum level was lower than the mean value of SP-D in the H1N1 cases (39.33 ng/mL vs 434.5 ng/mL) that were studied by Delgado et al. This may be caused by the pathophysiological process of COVID-19 itself, whereas it is known that SARS CoV-2 enters the respiratory tract and binds to the ACE-2 receptor to enter type II alveolar cells. Type II alveolar cells’ function is to produce surfactant, so the damage to these cells early in the course of the disease can decrease the amount of pulmonary surfactant. This may be the reason why the concentration of SP-D that leaks into the systemic circulation is not as high as with H1N1 infection.
Correlation between SP-D Serum Levels and ARDS Severity in COVID-19 Patients
A study conducted by Kerget et al. showed that in COVID-19 patients, day 0 SP-D levels were higher in patients with ARDS than without ARDS (P=0.001). However, the study by Kerget et al. only divided the patient groups into ARDS and non-ARDS, while the severity of ARDS was not elaborated. The study by Saito et al. showed that the SP-D serum levels showed a significant difference between the mild disease group (non-ARDS) and the severe disease group (ARDS) (P<0.001). SP-D serum level examinations were performed serially on days 3, 5 and 8. The increase in SP-D serum levels on day 8, when compared to day 3, was 8.5 times higher, and was also accompanied by the worsening of the clinical condition.
The results of our study showing that there was a significant correlation between SP-D serum levels and the severity of ARDS, but with weak correlation strength, are slightly different to the research by Kerget et al. and Saito et al. This may be due to differences in the characteristics of the study subjects (especially the type of race and comorbidities), and the day of SP-D serum collection. The study by Park et al. concluded that high SP-D serum levels within 48 hours of ICU admission serve as a diagnostic marker for ARDS and this is supported by the study by Saito et al., who found that the SP-D serum levels on day 8 had increased by about 8.5 times when compared to day 3 (17, 18). In our study, SP-D serum levels were measured on day 0, so that this could be the cause of the weak correlation strength.
In our study, 4 samples were found to be outliers. Of these 4 samples, a high SP-D value was obtained but the type of ARDS suffered was mild or moderate. This result contradicts the research by Kerget et al. and Saito et al. This may be due to the comorbidities suffered by these 4 patients who had allergies. The presence of an inflammatory process and injury to lung tissue affects the process of synthesis and secretion of SP-D from lung epithelial cells to the systemic circulation (19).
Koopmans et al. found an increase in SP-D levels in allergic patients compared to control subjects. SP-D levels increased 24 hours after allergen exposure in asthmatic patients, and were positively correlated with the number of eosinophils in the sputum. Thus, SP-D serum levels can serve as markers of the level of bronchial inflammation in allergic patients(20). Inhaled allergens are initially dissolved in the fluid lining the airways before they come into contact with immune cells. The airway lining fluid contains surfactant, and therefore the initial contact between the allergen and the surfactant component will occur earlier. SP-A and SP-D interact with mite allergens via CRD, and inhibit the binding of allergen-specific IgE to mite allergens. This finding may indicate that SP-D inhibits the induction of allergic reactions by direct allergen binding, and thus is beneficial in reducing the binding of dendritic cells and allergen-specific IgE, thereby preventing acute asthma attacks(21).
Correlation between SP-D Serum Levels with Mortality in COVID-19 Patients
The analysis results of this study showed that there was no significant correlation between SP-D serum levels on day 0 with mortality. This result is different from the study by Kerget et al., where the SP-D serum levels in the group that died (7 patients) compared to those who survived (81 patients) were 96.7±37.2 ng/ml and 56.9±43.5 ng/ml (P=0.03) respectively (22).
The report by Ruan et al. on 68 COVID-19 patients who died in Wuhan showed that 53% died due to respiratory failure, 7% due to shock (possibly due to fulminant myocarditis), 33% due to both, and 7% from unclear mechanisms (23). Elezkurtaj et al. conducted an autopsy study on 26 COVID-19 patients who died in a Berlin hospital, with the aim of finding the exact causes of the deaths of these patients. The autopsy results found that the most common immediate causes of death were septic shock and/or multi-organ failure (30.8%), viral pneumonia with or without signs of bacterial superinfection (19.2%), respiratory failure due to diffuse alveolar damage or ARDS (19.2%), right ventricular heart failure (15.4%), and massive pulmonary thromboembolism, severe bronchial aspiration, gastrointestinal bleeding, or left ventricular heart failure (3.8% each). These two studies show that the most common causes of death in COVID-19 patients are ARDS and septic shock accompanied by multi-organ failure (24).
SP-D serum levels, as a pulmonary epithelial marker, have been shown to correlate with ARDS mortality in COVID-19 patients (25). Early identification of pulmonary epithelial injury is one way of detecting the early stages of Acute Lung Injury before it deteriorates to ARDS (19). SP-D serum levels are thought to be a more specific marker for the damage that occurs in the lungs, but they are not specific for the damage that occurs in other organs.
In addition to the role of SP-D as a biomarker capable of describing the severity of ARDS in COVID-19, some opinions support the possibility that surfactant therapy may provide benefits in COVID-19 cases. Although pulmonary surfactant therapy is a standard, safe and effective therapy for neonates with ARDS, treatment with recombinant SP-C-based surfactant has not shown an increase in survival in a randomized controlled trial in adults (26). The use of natural surfactants seems to be more advantageous than synthetic surfactants in increasing blood oxygenation significantly and shortening ventilatory time in infant patients. Meconium aspiration syndrome resembles COVID-19 pneumonia in which there is decreased surfactant production due to the destruction of type II alveolar cells. Early administration of natural surfactants reduces the need for ECMO therapy and ventilatory time in infant patients. This suggests that initial administration of natural surfactants should also improve lung function in adult patients with severe ARDS. Thus, surfactant therapy in ARDS patients due to COVID-19 may be of benefit, especially when applied early in the disease course (Figure 1).
Figure 1.
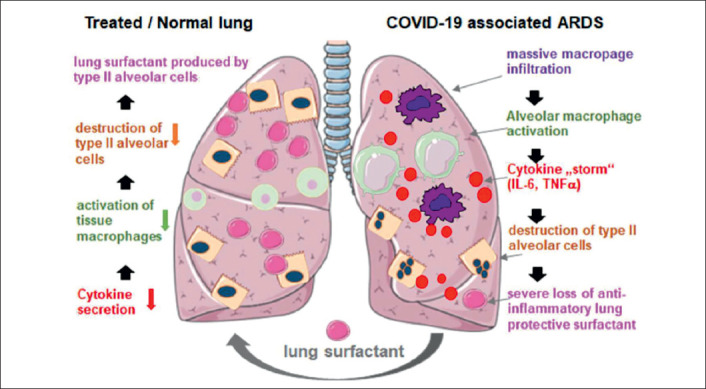
Hypothesis of Surfactant Therapy Effect Mechanism.
There are not many studies about the correlation between SP-D serum levels with ARDS severity and mortality in COVID-19 cases, so further research still needs to be done. The limitations of this study were the relatively low number of patients and the lack of adjustment for confounders. Using a control group and SP-D serum levels on collected serial days may be able to provide a better picture of the correlation between SP-D serum levels and the ARDS severity in COVID-19 patients.
Conclusion
From this study, it can be concluded that SP-D serum levels had a significant and weak correlation strength with ARDS severity, but not a significant correlation with mortality.
Authors’ Contributions:
Conception: AA and ASV; Design: AA, ASV, and AU; Acquisition, analysis and interpretation of data: AA, ASV, and AU; Drafting the article: AA; Revising it critically for important intellectual content: AA, ASV, and AU; Approved final version of the manuscript: AA, ASV, and AU.
Footnotes
What Is Already Known on This Topic:
In our study, it was found that the mean serum level of SP-D in the survivors was 38.93 ng/mL, whereas in the deceased group it was 39.7 ng/mL. This indicates that there is no significant relationship between serum SP-D levels on day 0 with mortality (P= 0.89). This result is different from a previous study which stated that the serum SP-D levels in the dead group (7 patients) compared to the survivor group (81 patients) were 96.7 ± 37.2 ng/ml and 56.9 ± 43.5 ng. / ml (P= 0.03). Then, serum SP-D levels were considered as a more specific marker for lung damage, but not specific for other organ damage.
What This Study Adds:
The study used an analytic observational perspective with a prospective cohort study design, in which the study population were COVID-19 infection patients suffering from COPD, Asthma and TB infection; patients with autoimmune disease, immunocompromised or taking immunosuppressant drugs; and patients with malignancy or receiving chemotherapy treatment.
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis Campaign:guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020;46(5):854–87. doi: 10.1007/s00134-020-06022-5. doi:10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phua J, Weng L, Ling L, Egi M, Lim CM, Divatia JV, et al. Intensive care management of coronavirus disease 2019 (COVID-19):challenges and recommendations. Lancet Respir Med. 2020;8(5):506–17. doi: 10.1016/S2213-2600(20)30161-2. doi:10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goh KJ, Choong MC, Cheong EH, Kalimuddin S, Duu Wen S, Phua GC, et al. Rapid Progression to Acute Respiratory Distress Syndrome:Review of Current Understanding of Critical Illness from Coronavirus Disease 2019 (COVID-19) Infection. Ann Acad Med Singap. 2020;49(3):108–18. [PubMed] [Google Scholar]
- 4.Spadaro S, Fogagnolo A, Campo G, Zucchetti O, Verri M, Ottaviani I, et al. Markers of endothelial and epithelial pulmonary injury in mechanically ventilated COVID-19 ICU patients. Crit Care. 2021;25(1):74. doi: 10.1186/s13054-021-03499-4. doi:10.1186/s13054-021-03499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vieceli Dalla Sega F, Fortini F, Spadaro S, Ronzoni L, Zucchetti O, Manfrini M, et al. Time course of endothelial dysfunction markers and mortality in COVID-19 patients:A pilot study. Clin Transl Med. 2021;11(3):e283. doi: 10.1002/ctm2.283. doi:10.1002/ctm2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartl D, Griese M. Surfactant protein D in human lung diseases. Eur J Clin Invest. 2006;36(6):423–35. doi: 10.1111/j.1365-2362.2006.01648.x. doi:10.1111/j.1365-2362.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- 7.Delgado C, Krötzsch E, Jiménez-Alvarez LA, Ramírez-Martínez G, Márquez-García JE, Cruz-Lagunas A, et al. Serum Surfactant Protein D (SP-D) is a Prognostic Marker of Poor Outcome in Patients with A/H1N1 Virus Infection. Lung. 2015;193(1):25–30. doi: 10.1007/s00408-014-9669-3. doi:10.1007/s00408-014-9669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yustinawati R, Achadi A. Risk Factors for Mortality in Patients with COVID-19:A Systematic Review. The 7th International Conference on Public Health, Solo, Indonesia, November 18-19, 2020 |71. doi:https://doi.org/10.26911/the7thicph.01.26. [Google Scholar]
- 9.Ortona E, Pierdominici M, Rider V. Editorial:Sex Hormones and Gender Differences in Immune Responses. Front Immunol. 2019;10:1076. doi: 10.3389/fimmu.2019.01076. doi:10.3389/fimmu.2019.01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bastolla U. Mathematical Model of SARS-Cov-2 Propagation Versus ACE2 Fits COVID-19 Lethality Across Age and Sex and Predicts That of SARS. Front Mol Biosci. 2021;8:706122. doi: 10.3389/fmolb.2021.706122. doi:10.3389/fmolb.2021.706122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Booth A, Reed AB, Ponzo S, Yassaee A, Aral M, Plans D, et al. Population risk factors for severe disease and mortality in COVID-19:A global systematic review and meta-analysis. PLoS One. 2021;16(3):e0247461. doi: 10.1371/journal.pone.0247461. doi:10.1371/journal.pone.0247461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo H, Liu S, Wang Y, Phillips-Howard PA, Ju S, Yang Y, et al. Age differences in clinical features and outcomes in patients with COVID-19, Jiangsu, China:a retrospective, multicentre cohort study. BMJ Open. 2020;10(10):e039887. doi: 10.1136/bmjopen-2020-039887. doi:10.1136/bmjopen-2020-039887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Contoli M, Papi A, Tomassetti L, Rizzo P, Vieceli Dalla Sega F, Fortini F, et al. Blood Interferon-αLevels and Severity, Outcomes, and Inflammatory Profiles in Hospitalized COVID-19 Patients. Front Immunol. 2021;12:648004. doi: 10.3389/fimmu.2021.648004. doi:10.3389/fimmu.2021.648004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roncon L, Zuin M, Rigatelli G, Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol. 2020;127:104354. doi: 10.1016/j.jcv.2020.104354. doi:10.1016/j.jcv.2020.104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho W-P, Liau JJ, Cheng CK. Biomechanical study of bone-patellar tendon-bone and bone-ACL-bone grafts. J Med Biol Eng. 2002;22(2):103–8. [Google Scholar]
- 16.Zhang H, Zhang Y, Wu J, Li Y, Zhou X, Li X, et al. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg Microbes Infect. 2020;9(1):1958–64. doi: 10.1080/22221751.2020.1812437. doi:10.1080/22221751.2020.1812437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang MC, Park YK, Kim BO, Park D. Risk factors for disease progression in COVID-19 patients. BMC Infect Dis. 2020;20(1):445. doi: 10.1186/s12879-020-05144-x. doi:10.1186/s12879-020-05144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saito A, Kuronuma K, Moniwa K, Kodama K, Takahashi S, Takahashi H, et al. Serum surfactant protein A and D may be novel biomarkers of COVID-19 pneumonia severity. Research Square. 2020;May:1–17. [preprint] [Google Scholar]
- 19.Veterini AS. A primary biomarker examination in preventing progressivity of acute respiratory distress syndrome:The role of surfactant protein-d in sepsis induced ARDS. Crit Care Shock. 2020;23(2):65–75. [Google Scholar]
- 20.Koopmans JG, van der Zee JS, Krop EJ, Lopuhaä CE, Jansen HM, Batenburg JJ. Serum surfactant protein D is elevated in allergic patients. Clin Exp Allergy. 2004;34(12):1827–33. doi: 10.1111/j.1365-2222.2004.02083.x. doi:10.1111/j.1365-2222.2004.02083.x. [DOI] [PubMed] [Google Scholar]
- 21.Hohlfeld JM, Erpenbeck VJ, Krug N. Surfactant proteins SP-A and SP-D as modulators of the allergic inflammation in asthma. Pathobiology. 2002-2003. 70(5):287–92. doi: 10.1159/000070744. doi:10.1159/000070744. [DOI] [PubMed] [Google Scholar]
- 22.Kerget B, Kerget F, Koçak AO, Kızıltunç A, Araz Ö, Uçar EY, et al. Are Serum Interleukin 6 and Surfactant Protein D Levels Associated with the Clinical Course of COVID-19? Lung. 2020;198(5):777–84. doi: 10.1007/s00408-020-00393-8. doi:10.1007/s00408-020-00393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–8. doi: 10.1007/s00134-020-05991-x. doi:10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elezkurtaj S, Greuel S, Ihlow J, Michaelis EG, Bischoff P, Kunze CA, et al. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci Rep. 2021;11(1):4263. doi: 10.1038/s41598-021-82862-5. doi:10.1038/s41598-021-82862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spadaro S, Park M, Turrini C, Tunstall T, Thwaites R, Mauri T, et al. Biomarkers for Acute Respiratory Distress syndrome and prospects for personalised medicine. J Inflamm. 2019;16(1) doi: 10.1186/s12950-018-0202-y. doi:https://doi.org/10.1186/s12950-018-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng SS, Chang W, Lu ZH, Xie JF, Qiu HB, Yang Y, et al. Effect of surfactant administration on outcomes of adult patients in acute respiratory distress syndrome:a meta-analysis of randomized controlled trials. BMC Pulm Med. 2019;19(1):9. doi: 10.1186/s12890-018-0761-y. doi:10.1186/s12890-018-0761-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


