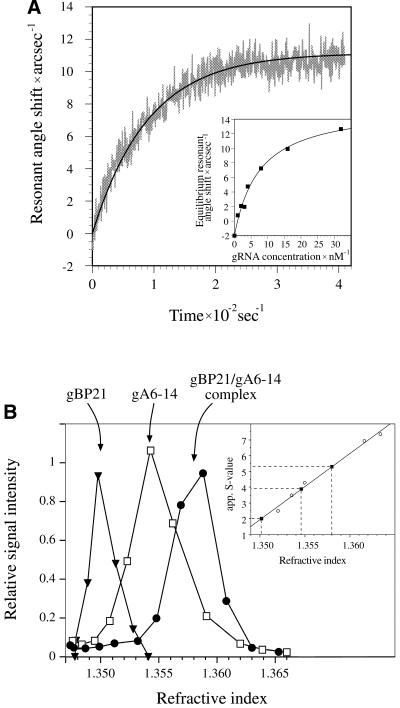
Figure 1.
Characterization of the gBP21/gRNA complex. (A) Determination of the kinetic and thermodynamic stability of the gBP21/gRNA complex using an optical biosensor (Affinity Sensors). gBP21 was covalently coupled to the surface of a micro-cuvette and the progress of the interaction with added gRNA gA6-14 was recorded in real time. The plot shows the change in the resonant angle shift as a function of time. A monoexponential curve fit of the data points allowed the determination of first order association rate constants. (Insert) Equilibrium signals were plotted as a function of the gRNA concentration and curve fitted. The obtained equilibrium dissociation constants are identical to the results obtained by adsorption filtration (Kd = 8 ± 2 nM) (12). The kinetic data were used to calculate the half-lives of three gBP21/gRNA complexes (see Table 1). (B) Determination of apparent sedimentation coefficients for gBP21, radioactively labeled gRNA (gA6-14) and the gA6-14/gBP21 complex. The three molecules were sedimented in linear glycerol gradients and plotted as a function of the refractive index of the gradient fractions. In comparison to the sedimentation behavior of standard proteins (insert) the following apparent S-values were derived: gBP21, 2.0 S; gRNA gA6-14, 3.9 S; gA6-14/gBP21 complex, 5.3 S. The apparent S-value of the complex was used to calculate the axial ratio of the gBP21/gRNA complex (see Materials and Methods), in agreement with a 1 + 1 stoichiometry.