Abstract
Background:
Meningitis causes significant mortality in sub-Saharan Africa and limited diagnostics exist. We evaluated the utility of the BioFire® FilmArray® Meningitis/Encephalitis multiplex PCR panel (BioFire ME) in HIV-infected adults and children presenting with suspected meningitis in Uganda.
Methods:
We tested cerebrospinal fluid (CSF) using a stepwise meningitis diagnostic algorithm including BioFire ME. We determined the diagnostic performance of BioFire ME for cryptococcal meningitis, using cryptococcal antigen (CrAg) and CSF culture as reference standards, and assessed other central nervous system (CNS) pathogens identified by the panel.
Results:
We evaluated 328 adult and 42 pediatric CSF specimens using BioFire ME. Of the adult CSF samples tested, 258 were obtained at baseline, and 70 were obtained from repeat lumbar punctures in cryptococcal meningitis. For Cryptococcus, sensitivity was 82%, specificity was 98%, PPV was 98%, and NPV was 79% in baseline specimens using CSF CrAg as the reference standard. Among follow-up specimens, a negative BioFire ME for Cryptococcus predicted CSF culture sterility with 84% NPV. Overall sensitivity was decreased at low fungal burdens: 29% for 0–99 Cryptococcus CFU/mL compared to 94% for ≥100 CFU/mL in baseline specimens. Other pathogens detected included E. coli, H. influenzae, S. pneumoniae, CMV, enterovirus, HSV, HHV-6, and VZV. Two specimens tested positive for S. pneumoniae and one for Cryptococcus in the pediatric population.
Conclusions:
Multiplex PCR is a promising rapid diagnostic test for meningitis in adults and children in resource-limited settings. Cryptococcus at low fungal burdens in CSF can be missed by BioFire ME.
Keywords: Uganda, meningitis, Multiplex PCR, HIV, cryptococcal meningitis, sensitivity
Introduction
Meningitis causes significant morbidity and mortality in sub-Saharan African.1 Meningitis caused by Cryptococcus neoformans is the leading cause of adult meningitis in this region and a major contributor to HIV-related mortality, responsible for approximately 15% of AIDS-related deaths.2 Viral, bacterial and parasitic infections also cause deadly and debilitating central nervous system (CNS) infections in people worldwide.3 Bacterial meningitis is associated with high mortality and the risk of long-term, major sequelae is highest in low-income countries and in Africa.4 In children living in malaria endemic regions, cerebral malaria can further obscure a meningoencephalitis clinical picture. Distinguishing cerebral malaria from other CNS infections is essential for rapid and effective treatment initiation.1
The diagnosis of cryptococcal meningitis is straight-forward and inexpensive where the cryptococcal antigen (CrAg) lateral flow assay (LFA) is available.5 Diagnosis of other causes of meningitis relies upon correlation of clinical presentation with routine and specialized CSF testing. Specialized testing, including serology or PCR-based testing for individual pathogens, is not frequently available in African settings. One exception is the GeneXpert MTB/RIF, a cartridge-based assay for Mycobacterium tuberculosis available throughout much of Africa through a concessional pricing scheme.6
Despite the introduction of the CrAg-LFA and Xpert MTB/RIF, rapid and affordable diagnosis of meningitis remains challenging, especially in resource-limited settings. In 2017, the WHO officially released a callout to biotechnology companies to develop rapid diagnostic tests (RDTs) for meningitis.7 Multiplex PCR-based testing for infectious diseases has become commonplace in high-income countries and is emerging in low-income countries.8 The effectiveness of Xpert MTB/RIF and its utility in developing countries demonstrates the potential of PCR-based testing in resource-limited settings. We evaluated the clinical utility of the FDA-approved, multiplex PCR-based commercial assay (the BioFire® FilmArray® Meningitis/Encephalitis (ME) panel; BioFire Diagnostics, Salt Lake City, UT, USA) which tested cerebrospinal fluid (CSF) on-site in Ugandan for two cohorts of adults and children presenting with suspected meningitis.
Materials and methods
This study was conducted in part as a diagnostic sub-study for the Adjunctive Sertraline for the Treatment of HIV-Associated Cryptococcal Meningitis (ASTRO-CM) trial.9 ASTRO-CM was a phase III randomized, double blind, placebo-controlled trial to evaluate whether adjunctive sertraline would improve survival in cryptococcosis. This study included HIV-infected adults with suspected meningitis screened with a diagnostic lumbar puncture. Diagnosis of cryptococcal meningitis was made by CrAg-LFA and later confirmed by CSF fungal culture. Those with confirmed cryptococcal meningitis underwent therapeutic lumbar punctures (LPs) on days 3, 7, and 10–14 of amphotericin therapy and additionally as clinically required for intracranial pressure control.
We tested baseline CSF samples collected at recruitment to determine the performance of the BioFire ME for diagnosing cryptococcal meningitis compared to CSF CrAg. To further investigate BioFire ME’s ability to detect Cryptococcus at lower fungal burdens and to determine correlation of a negative result with reversion to culture sterility, we tested CSF from a subset of follow-up therapeutic lumbar punctures among adults receiving treatment for cryptococcal meningitis; for these repeat CSF samples we used CSF culture as the reference standard. Additionally, both HIV-infected and uninfected children presenting with signs and symptoms suggestive of CNS infection, such as cerebral malaria and/or meningitis, were included as a separate analysis.
Setting and individuals
From September 2016 to April 2019, we recruited HIV-infected adults presenting with suspected meningitis to Mulago National Referral Hospital and Mbarara Regional Referral Hospital in Uganda. Participants were investigated for infective meningitis using a stepwise diagnostic algorithm (Supplemental Figure 1). For the pediatric cohort, we recruited children aged 0–17 years admitted to the Mbarara pediatric ward from July 2017 to October 2018. We collected clinical data in real-time from existing patient charts using an intake form. If the child received a procedure or laboratory testing as part of their routine care, these data were recorded.
All participants (or surrogate) provided written informed consent for lumbar puncture (LP), CSF testing and storage, and data collection. Institutional review boards in Uganda and at the University of Minnesota approved the study. BioFire ME cartridges were donated by BioFire Diagnostics at no cost as part of investigator-initiated research. BioFire Diagnostics played no role in the collection, analysis, or interpretation of study data.
CSF samples and examination
CrAg testing was performed on all CSF specimens, and Xpert MTB/RIF was performed on CrAg-negative CSF specimens. Cryptococcal meningitis was diagnosed immediately at the bedside using CrAg-LFA (Immy, Norman, Oklahoma). All routine CSF and blood testing was performed locally, and included CSF white cell count, protein, glucose, staining and bacterial cultures.10 Quantitative CSF fungal cultures were performed using Sabouraud Dextrose Agar (SDA) on fresh CSF with five 1:10 serial dilutions of 100 μL of CSF.11 A positive culture was defined as any growth of Cryptococcus after 10 days. Mycobacterial culture using solid and liquid media, and tuberculosis testing using Xpert MTB/RIF Ultra were performed on CrAg-negative CSF specimens. CSF analyzed by BioFire ME was on site, prospectively in real time per manufacturer’s instructions.
In the pediatric cohort, BioFire ME was performed on all CSF samples collected from the Mbarara pediatric ward, obtained per clinician judgment as part of diagnostic workup for children presenting with symptoms of meningitis. Additional laboratory testing including complete blood count, blood chemistries, malaria rapid direct test, and rapid HIV testing were performed on individuals per clinician discretion.
Statistical analyses
We used CSF from baseline LPs to calculate the sensitivity and specificity of BioFire ME for identification of Cryptococcus in adult patients. We used CrAg-LFA as the reference standard in patients presenting with their first episode of cryptococcal meningitis, and fungal culture in patients with a previous history of cryptococcosis. We assessed BioFire ME’s ability to detect Cryptococcus over the treatment course by analyzing additional adult specimens obtained by therapeutic LPs 2–28 days after cryptococcal diagnosis. We calculated positive and negative predictive values compared to CSF fungal culture (reference standard). Additional outcomes included detection of other CNS pathogens by BioFire ME. Except for Cryptococcus, no reference standards were used to confirm the presence of other pathogens.
Results
Adult Cohort
We prospectively collected and tested 328 adult CSF specimens using BioFire ME. Of all CSF samples collected, 258 specimens (79%) were collected at hospital presentation (baseline tests). Of these baseline tests, 106 (41%) were collected from CrAg-negative individuals, and 152 (59%) CSF specimens were collected from CrAg-positive individuals of whom 11 were presenting with second symptomatic episode with differential diagnosis of culture-positive relapse versus paradoxical immune reconstitution inflammatory syndrome. Table 1 describes the demographics, clinical characteristics and baseline CSF analysis results, stratified by CSF CrAg status.
Table 1:
Baseline demographics of adult HIV+ cohort by CSF CrAg status.
| CSF CrAg+ | CSF CrAg- | |
|---|---|---|
| N=152 | N=106 | |
| Demographics | ||
| Age, years | 34 [30; 40] | 34 [29; 42] |
| Women | 55 (36%) | 52 (49%) |
| Weight, kg | 51 [49; 59] | 53.5 [50; 60] |
| Clinical Characteristics | ||
| Glasgow coma scale < 15 | 70 (46%) | 76 (71%) |
| Duration of HIV, months | 14.1 [0.5, 49.4] | 3.7 [0.2, 32.1] |
| CD4 cell count, cells/mm3 | 15 [6; 47] | 58 [19; 170] |
| Receiving ART | 79 (52%) | 56 (54%) |
| Baseline CSF Analysis Results | ||
| Opening Pressure, cm H2O | 27 [19; 40] | 14 [9.5; 23] |
| Cryptococcus CFU/mL | 100,500 [1,100; 520,000] | 0 |
| CSF white cells ≥5/μL | 56 (39%) | 35 (35%) |
| CSF Protein, mg/dL | 40 [23; 88] | 37 [22; 100] |
Data are N (%) or medians with [P25, P75].
Abbreviations: CSF, cerebrospinal fluid; CrAg, cryptococcal antigen; CFU, colony-forming units.
Of baseline specimens tested by BioFire ME, 41% (105/258) had no pathogen detected; 41% (106/258) detected Cryptococcus only; 8% (21/258) detected Cryptococcus and another pathogen, and 10% (26/258) detected another pathogen only (Table 2). Cryptococcus was detected by BioFire ME in 82% (125/152) of CSF CrAg-positive CSF, and in 2% (2/106) of CSF CrAg-negative specimens. This yielded an overall BioFire ME Cryptococcus diagnostic sensitivity of 82%, a specificity of 98%, a positive predictive value of 98%, and a negative predictive value of 79%, when using CSF CrAg as the reference standard. One of the two false positives occurred in an individual with a positive serum CrAg but negative CSF CrAg, thus disseminated cryptococcosis with early meningitis was possible. Serum CrAg testing was not performed on the other false positive CSF sample.
Table 2:
Results and diagnostic performance of BioFire ME in baseline and diagnostic CSF samples.
| Baseline specimens | Follow-up specimens | |||||
|---|---|---|---|---|---|---|
| Cryptococcal Meningitis* | Cryptococcal Meningitis† | |||||
| Yes | No | Overall | Yes | No | Overall | |
| Summary of FilmArray ME results | ||||||
| No pathogen detected | 23 | 82 | 105 | 5 | 21 | 26 |
| Cryptococcus only by FilmArray ME | 104 | 2 | 106 | 22 | 10 | 32 |
| Cryptococcus and another pathogen | 21 | 0 | 21 | 3 | 4 | 7 |
| Another pathogen only | 4 | 22 | 26 | 0 | 5 | 5 |
| Overall | 152 | 106 | 258 | 30 | 40 | 70 |
| Summary of Cryptococcus results | ||||||
| Cryptococcus detected by BioFire ME | 125 | 2 | 127 | 25 | 14 | 39 |
| Cryptococcus not detected by BioFire | 27 | 104 | 131 | 5 | 26 | 31 |
| Overall | 152 | 106 | 258 | 30 | 40 | 70 |
| Diagnostic performance for Cryptococcus | ||||||
| Sensitivity P (T+ | D+) | 0.82 | 0.83 | ||||
| Specificity P (T- | D-) | 0.98 | 0.65 | ||||
| Positive Predictive Value P (D+ | T+) | 0.98 | 0.64 | ||||
| 0.79 | 0.84 | |||||
CSF CrAg at time of diagnosis used as gold standard in baseline specimens
CSF quantitative culture > 0 CFU/mL used as gold standard for follow-up (days 2–28) specimens; with the clinical question being whether a negative PCR predicts a negative culture (84% negative predictive value).
The sensitivity of BioFire ME in baseline CSF samples was dependent on fungal burden, with lower assay sensitivity at low fungal burdens. Of the 27 BioFire ME false negatives in baseline specimens: 52% (14/27) were from specimens with sterile CSF cultures (no growth in CSF cultures despite having a positive CSF CrAg) and the BioFire ME sensitivity among samples with sterile CSF cultures was only 13% (2/16). While the overall sensitivity was improved using CSF cultures rather than CSF CrAg as reference standard, the sensitivity among baseline CSF samples with positive growth of Cryptococcus was notably dependent on fungal burden. The overall sensitivity among those with positive baseline CSF cultures was 91% (122/134); sensitivity for fungal burden < 100 CFU/mL and ≥100 CFU/mL was 29% (2/7) and 94% (119/127), respectively (Figure 1).
Figure 1. Sensitivity of BioFire ME among baseline CSF samples, by initial Cryptococcus burden.
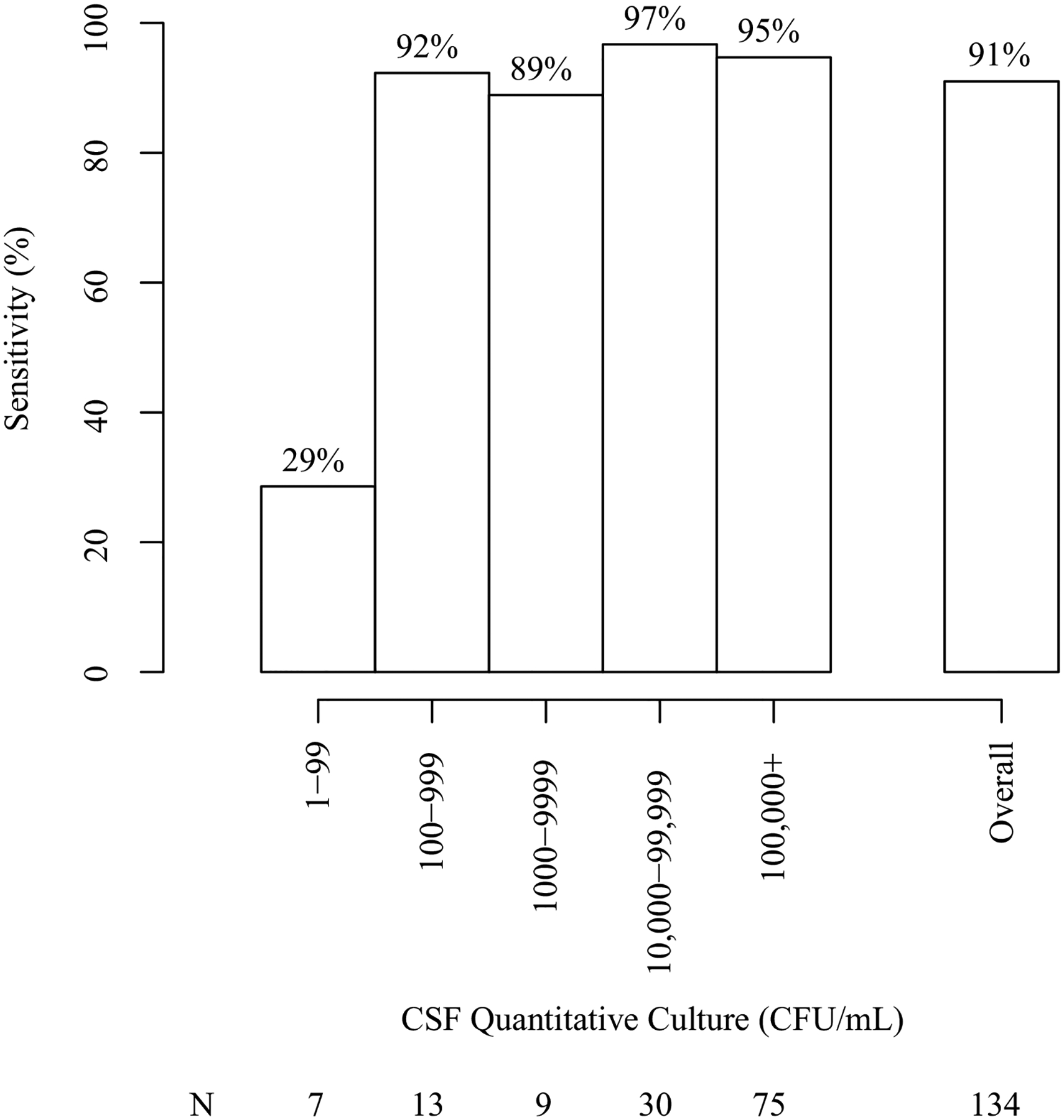
The sensitivity of BioFire ME for Cryptococcus was low in initial diagnostic CSF samples with low quantitative culture growth (<100 CFU/mL). Most CSF samples in this population generally presented with very high initial fungal burdens (≥100,000 CFU/mL). Of persons with CSF CrAg positive only with sterile cultures, Biofire ME was positive in 12.5% (2/16), excluding two persons with paradoxical IRIS.
In 11 individuals presenting with symptoms of meningitis and a previous history of Cryptococcus (symptomatic relapse), BioFire ME identified 89% (8/9) of cases of culture-positive fungal relapse and was negative for Cryptococcus in both cases having sterile cultures. Thus BioFire ME correctly classified culture status in 10 of 11 persons with second episode of cryptococcal meningitis.
Of the 70 CSF specimens that were collected from therapeutic lumbar punctures performed 2–28 days after cryptococcal diagnosis (Table 2), 30 had positive CSF cryptococcal cultures and 40 had sterile CSF cultures. Cryptococcus was detected by multiplex PCR in 83% (25/30) of specimens with positive CSF cultures and was not detected in 65% (26/40) specimens with corresponding sterile cultures. BioFire ME predicted conversion to culture sterility with 84% (26/31) negative predictive value (when BioFire ME was negative for Cryptococcus, culture was sterile).
In total, 328 CSF samples were collected and tested from 315 individuals. Thirteen individuals had both a baseline and follow-up test. The sensitivity of BioFire ME in pooled specimens (both baseline diagnostic samples and follow-up samples from those with cryptococcosis) using CSF culture as a reference standard was 81% (Supplemental Table 1) and was still dependent on fungal burden, with lower sensitivity of the assay at low fungal burdens (Supplemental Figure 2).
A total of 47 individuals had a total of 55 other (non-Cryptococcus) pathogens detected by BioFire ME in baseline CSF. This data is further described in Table 3, with listing of pathogens detected by cryptococcal status. The most common pathogens detected were human herpes virus 6 (N=12), Streptococcus pneumoniae (N=11), cytomegalovirus (N=11), and varicella-zoster virus (N=7). Except for S. pneumoniae, which was more common in patients without cryptococcosis, the frequency that other pathogens were detected in CSF was similar in patients with and without Cryptococcus.
Table 3:
Results and diagnostic performance of BioFire ME in baseline CSF samples.
| Concurrent cryptococcal meningitis | Non-cryptococcal meningitis | Overall | ||||
|---|---|---|---|---|---|---|
| (N=152) | (N=106) | (N=258) | ||||
| Number | Frequency | Number | Frequency | Number | Frequency | |
| Escherichia coli K1 | 0 | 0.0% | 1 | 0.9% | 1 | 0.4% |
| Haemophilus influenzae | 2* | 1.3% | 1 | 0.9% | 3 | 1.2% |
| Streptococcus pneumoniae | 3* | 2.0% | 8 | 7.5% | 11 | 4.3% |
| Cytomegalovirus | 6 | 4.0% | 5 | 4.7% | 11 | 4.3% |
| Enterovirus | 1 | 0.7% | 0 | 0.0% | 1 | 0.4% |
| Herpes simplex virus 1 | 0 | 0.0% | 4 | 3.8% | 4 | 1.6% |
| Herpes simplex virus-2 | 3 | 2.0% | 2 | 1.9% | 5 | 1.9% |
| Human herpesvirus 6 | 7 | 4.6% | 5 | 4.7% | 12 | 4.7% |
| Varicella zoster virus | 4 | 2.6% | 3 | 2.8% | 7 | 2.7% |
| Total number of pathogens | 26 | 29 | 55 | |||
Probable contamination during collection. Subsequent wearing of surgical masks during collection, eliminated further respiratory pathogen detection.
Pediatric Cohort
In the pediatric population, a total of 42 CSF specimens were prospectively collected by baseline LP. The pediatric cohort consisted of 41% (17/42) females with a median age of 46 months (3.8 years). Of those tested by BioFire ME, 14% (6/42) were HIV-positive, 19% (8/42) were malaria-positive (either by RDT or smear), and 36% (15/42) were taking antibiotics upon arrival to hospital. S. pneumoniae was detected in 2 (5%) samples, and Cryptococcus was detected in 1 (3%) sample (also serum CrAg positive; no CSF CrAg obtained). Routine CSF testing and BioFire ME testing were performed on all 42 specimens. Elevated CSF protein and elevated CSF white count suggestive of meningitis was detected in only 2 (5%) specimens (Supplemental Table 2).
Of the eight children who tested positive for malaria, all had a negative BioFire ME result. Two children testing positive for S. pneumoniae were malaria negative and not taking antibiotics. Both children with elevated CSF protein and WBC count suggestive of meningitis were not taking antibiotics and tested negative by BioFire ME. These data are presented in Supplemental Table 3.
Discussion
This study demonstrates that while BioFire ME offers promise as a rapid diagnostic test for meningitis in specific clinical scenarios, its utility in cryptococcal meningitis is limited by poor sensitivity at low fungal burdens. Our data also demonstrates that the BioFire ME performs better using CSF cryptococcal cultures as a reference standard than it does using CSF CrAg as a reference standard. This observation is supported by two additional studies from the United States that evaluated N=37 and N=50 CSF CrAg-positive samples.12, 13 Thus, the poor sensitivity of BioFire ME for Cryptococcus in diagnostic specimens can be partially explained through cases of CSF culture-negative, CrAg-positive meningitis, which was more common than expected in this population. In contrast to previous studies which attributed the high number of false negative results to persistent CSF CrAg in the setting of previously treated cryptococcal meningitis, however, we excluded prior meningitis and assert that culture-negative CSF in our cohort represents early meningitis.14, 15 Thus, we feel that CSF CrAg was the appropriate reference standard in this study. Furthermore, we observed poor sensitivity of BioFire ME for Cryptococcus at low fungal burdens, even when using CSF cultures as a reference standard. Given the diagnostic importance of detecting Cryptococcus in CSF, we postulate that future PCR-based assays for cryptococcal meningitis would benefit from optimized sensitivity, even if at the expense of worse specificity. In the meantime, BioFire ME should not be a replacement for CrAg or cryptococcal culture in immunocompromised populations.
The high positive predictive value of BioFire ME that we observed for Cryptococcus suggests utility in detecting infection in patients with a low suspicion for cryptococcal disease, a finding that has been substantiated in a recent systematic review.16 In settings with lower HIV prevalence, for example, cryptococcal diagnosis may be fortuitous or delayed, and inclusion of Cryptococcus on a multiplex platform would allow for rapid recognition. Given the poor sensitivity of BioFire ME for Cryptococcus at low fungal burdens, however, we would advise caution when interpreting test results, especially in HIV-uninfected populations where the initial fungal burden is often low.17 In hospitals that have adopted multiplex PCR, a negative BioFire ME result does not rule out Cryptococcus. Standard assessment with CrAg and cultures should be performed whenever diagnosis remains elusive. This study provides further insight into recent cases of missed cryptococcal meningitis due to false-negative BioFire ME results.17–20 BioFire does not endorse the BioFire ME panel as a standalone test to be used in place of CrAG testing, as is described in the panel’s included technical note.
Despite the limitations in the diagnosis of cryptococcal meningitis, we nonetheless observed clinical utility of CSF PCR for cryptococcal disease and in measuring the response to anti-fungal treatment. Currently, monitoring relies primarily on CSF cultures (which take 7–10 days to result and are rarely available outside the research setting), and CrAg cannot be used to monitor response as it can remain positive in CSF for months to years after initial cryptococcal infection.21 In follow-up specimens from patients receiving treatment for cryptococcal meningitis, BioFire ME predicted conversion to culture sterility with a negative predictive value of 84%. This suggests that PCR positivity clears with resolution of culture-positivity. One possible application of PCR could therefore be customized duration of amphotericin treatment based on conversion to PCR negativity with treatment.22 A negative BioFire ME result could rapidly confirm either sterility or very low fungal burden after which amphotericin therapy could be discontinued, preventing its further toxic effects, shortening treatment length and reducing cost. This is considered off-label use of the panel and would require further study.
BioFire ME could also be useful in differentiating culture-positive relapse from other etiologies including paradoxical immune reconstitution inflammatory syndrome (IRIS) with sterile CSF cultures in patients presenting with recurrence of CNS symptoms. Currently, we must rely upon ruling out relapse with labor and time-consuming CSF cryptococcal cultures, which severely limits the clinical utility of cultures.22 Thus, a test capable of rapidly determining active infection would be an important advance in cases of recurrent disease, and use of the CSF PCR for this purpose warrants further investigation. Although our sample size was small, it is reasonable to conclude high specificity and high positive predictive value of the BioFire ME in this population.
This study is among the first to evaluate multiplex PCR-based testing for meningitis in children in a malaria-endemic, resource-limited setting. Although limited by low enrollment in the pediatric arm, we found that BioFire ME was helpful for excluding bacterial causes of meningitis and did note one HIV-positive child testing positive for Cryptococcus by BioFire ME (confirmed by serum CrAg), which indicates Cryptococcus can affect both adults and children. We also detected S. pneumoniae in two of the pediatric CSF samples. In both these cases, CSF cultures were negative despite the lack of antibiotics, and contamination was suspected. Contamination is always a consideration with molecular-based diagnostics, and these cases highlight the need to interpret positive PCR results in the clinical context.
Advantages to BioFire ME in our setting included ease of set up, minimal CSF processing, and multiple pathogen targets requiring a small sample volume, and detection of other viral pathogens not otherwise easily detected in this population. Disadvantages included cost, difficulty servicing the instrument, and operating the instrument on an inconsistent power grid. For power outages, a universal power supply (UPS) system solved this issue. This UPS was created using two 24-volt car batteries and a sine wave inverter, was made with local supplies and could be serviced by local electricians (Figure 2). Including a built-in UPS adaptable to large batteries would ensure continuous operation with variable power supplies.
Figure 2: Universal Power Supply for FilmArray ME instrument.
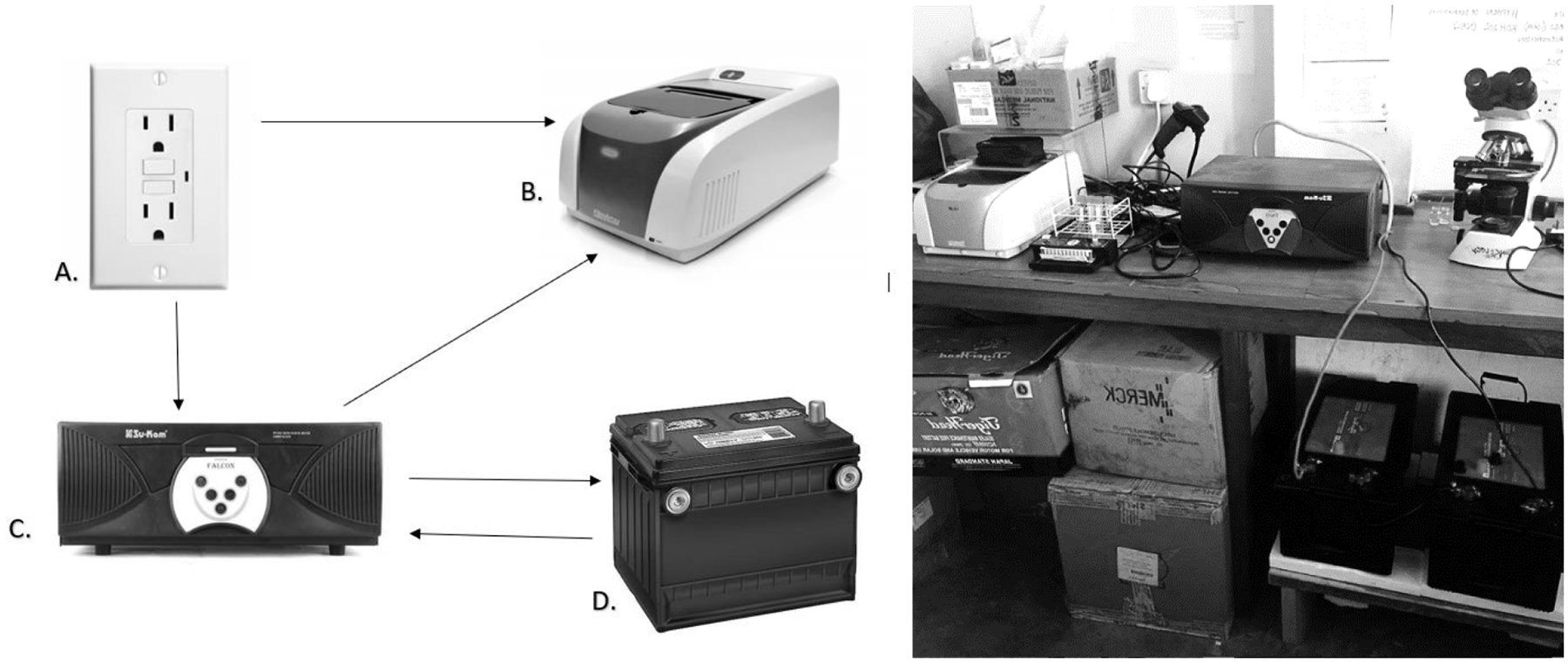
Arrows indicate flow of electricity. Wall socket (A) is connected to FilmArray ME instrument and a sine wave inverter (C). The sine wave inverter is connected to the FilmArray ME instrument and two 24-volt car batteries (D). When the FilmArray ME is not in use, power flows from the wall socket via the sine wave inverter to charge the batteries. During normal FilmArray ME operation, wall socket provides power directly to the instrument. In case of a power interruption, the sine wave inverter detects power interruption and instantly redirects power from the charged batteries to the FilmArray ME instrument. When wall socket re-establishes power supply, the sine wave inverter instantly redirects power from wall socket to recharge the batteries, and the wall socket returns to powering the FilmArray ME instrument directly.
Another disadvantage of BioFire ME is the inability to detect pathogens that might be expected in our immunosuppressed population. The addition of opportunistic pathogens such as M. tuberculosis, Toxoplasma gondii and John Cunningham (JC) virus to the panel would provide additional diagnostic utility in this population. Adding M. tuberculosis to the panel would be particularly useful as it is proposed to be the second-most common cause of meningitis in this population. Finally, the costs of the FilmArray platform will continue to limit its availability in sub-Saharan Africa unless efforts at subsidy or cost reduction are made.
Conclusion
BioFire ME is easy to use and implement, demonstrates potential utility in cryptococcal meningitis for monitoring treatment response and excluding relapse in cases of symptomatic recurrence. While PCR is useful in detecting Cryptococcus for these reasons, we observed poor sensitivity of the BioFire ME at low fungal burdens, and the costs were significantly higher than traditional testing by CrAg or cultures. Therefore, as described already in BioFire’s technical note on the panel, it cannot be recommended as a standalone diagnostic test for cryptococcal meningitis. BioFire ME was also useful for detecting viral pathogens, though the clinical significance of detectable virus in our immunocompromised population was often unclear. In immunosuppressed populations, the addition of common HIV-associated pathogens and M. tuberculosis would be optimal. In the pediatric population, the FilmArray ME may be helpful in differentiating febrile illness in children living in geographical regions with high malaria and meningitis burden. Despite several limitations, BioFire ME offers an efficient and promising means to rapidly and accurately diagnose meningitis globally in adults and children.
Supplementary Material
Acknowledgments
This research was supported by the United States Fogarty International Center (K01TW010268, R25TW009345), National Institute of Neurologic Diseases and Stroke (R01NS086312), National Institute of Allergy and Infectious Diseases (T32AI055433). This work was also supported in part by the Doris Duke Charitable Foundation through a grant supporting the Doris Duke International Clinical Research Fellows Program at the University of Minnesota. Sarah Bridge was a 2016–2017 Doris Duke International Clinical Research Fellow supported by the Doris Duke Research Foundation. RK and DM are supported through the DELTAS Africa Initiative grant # DEL-15-011 to THRiVE-2, from Wellcome Trust grant # 107742/Z/15/Z and the UK government. FVC is supported through a Wellcome Trust Clinical PhD Fellowship (Grant no. 210772/Z/18/Z). FVC is an honorary fellow of the Makerere University – Uganda Virus Research Institute Centre of Excellence for Infection and Immunity Research and Training (MUII-plus). MUII-plus is supported through the DELTAS Africa Initiative (Grant no. 107743). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS), Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (Grant no. 107743) and the UK Government. The MRC/UVRI and LSHTM Uganda Research Unit is jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement and is also part of the EDCTP2 program supported by the European Union.
Footnotes
Conflicts of Interest
All authors have no conflict of interest.
References
- 1.Singhi P, 2019. Central Nervous System Infections in Children: An Ongoing Challenge! Indian J Pediatr 86: 49–51. [DOI] [PubMed] [Google Scholar]
- 2.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR, 2017. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 17: 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2016 Neurology Collaborators, 2019. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18: 459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I, 2010. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis 10: 317–28. [DOI] [PubMed] [Google Scholar]
- 5.Kabanda T, Siedner MJ, Klausner JD, Muzoora C, Boulware DR, 2014. Point-of-care diagnosis and prognostication of cryptococcal meningitis with the cryptococcal antigen lateral flow assay on cerebrospinal fluid. Clin Infect Dis 58: 113–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO, 2010. WHO endorses new rapid tuberculosis test. Available at: https://www.who.int/mediacentre/news/releases/2010/tb_test_20101208/en/. Accessed 25 Sept, 2019.
- 7.WHO, 2017. Invitation to manufacturers of Meningitis rapid tests to submit an expression of interest. Available at: https://www.ungm.org/Public/Notice/62300. Accessed 25 Sept, 2019.
- 8.Rhein J, Bahr NC, Hemmert AC, Cloud JL, Bellamkonda S, Oswald C, Lo E, Nabeta H, Kiggundu R, Akampurira A, Musubire A, Williams DA, Meya DB, Boulware DR, Team A-C, 2016. Diagnostic performance of a multiplex PCR assay for meningitis in an HIV-infected population in Uganda. Diagn Microbiol Infect Dis 84: 268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhein J, Huppler Hullsiek K, Tugume L, Nuwagira E, Mpoza E, Evans EE, Kiggundu R, Pastick KA, Ssebambulidde K, Akampurira A, Williams DA, Bangdiwala AS, Abassi M, Musubire AK, Nicol MR, Muzoora C, Meya DB, Boulware DR, team A-C, 2019. Adjunctive sertraline for HIV-associated cryptococcal meningitis: a randomised, placebo-controlled, double-blind phase 3 trial. Lancet Infect Dis 19: 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis J, Bangdiwala AS, Cresswell FV, Rhein J, Nuwagira E, Ssebambulidde K, Tugume L, Rajasingham R, Bridge SC, Muzoora C, Meya DB, Boulware DR, 2019. The Changing Epidemiology of HIV-Associated Adult Meningitis, Uganda 2015–2017. Open Forum Infect Dis 6: ofz419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyal J, Akampurira A, Rhein J, Morawski BM, Kiggundu R, Nabeta HW, Musubire AK, Bahr NC, Williams DA, Bicanic T, Larsen RA, Meya DB, Boulware DR, Team A-CT, 2016. Reproducibility of CSF quantitative culture methods for estimating rate of clearance in cryptococcal meningitis. Med Mycol 54: 361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van TT, Kim TH, Butler-Wu SM, 2020. Evaluation of the Biofire FilmArray meningitis/encephalitis assay for the detection of Cryptococcus neoformans/gattii. Clin Microbiol Infect.26: 1375–79. [DOI] [PubMed] [Google Scholar]
- 13.Liesman RM, Strasburg AP, Heitman AK, Theel ES, Patel R, Binnicker MJ, 2018. Evaluation of a Commercial Multiplex Molecular Panel for Diagnosis of Infectious Meningitis and Encephalitis. J Clin Microbiol 56 :e01927–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ssebambulidde K, Bangdiwala AS, Kwizera R, Kandole TK, Tugume L, Kiggundu R, Mpoza E, Nuwagira E, Williams DA, Lofgren SM, Abassi M, Musubire AK, Cresswell FV, Rhein J, Muzoora C, Hullsiek KH, Boulware DR, Meya DB, Adjunctive Sertraline for Treatment of HIVaCMT, 2019. Symptomatic Cryptococcal Antigenemia Presenting as Early Cryptococcal Meningitis With Negative Cerebral Spinal Fluid Analysis. Clin Infect Dis 68: 2094–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ssebambulidde K, Skipper C, Rhein J, 2019. Culture-negative cryptococcal meningitis. Lancet Infect Dis 19: 929–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tansarli GS, Chapin KC, 2020. Diagnostic test accuracy of the BioFire(R) FilmArray(R) meningitis/encephalitis panel: a systematic review and meta-analysis. Clin Microbiol Infect 26: 281–290. [DOI] [PubMed] [Google Scholar]
- 17.Pilmis B, Bougnoux ME, Guery R, Senghor Y, Le Monnier A, Lanternier F, Bretagne S, Alanio A, Lortholary O, 2020. Failure of multiplex meningitis/encephalitis (ME) NAT during cryptococcal meningitis in solid organ recipients. Transpl Infect Dis: e13263. [DOI] [PubMed] [Google Scholar]
- 18.Lewis PO, Lanier CG, Patel PD, Krolikowski WD, Krolikowski MA, 2019. False negative diagnostic errors with polymerase chain reaction for the detection of cryptococcal meningoencephalitis. Med Mycol.58: 408–10. [DOI] [PubMed] [Google Scholar]
- 19.Chew KL, Lee CK, Cross GB, Lum LHW, Yan B, Jureen R, 2018. Culture-confirmed cryptococcal meningitis not detected by Cryptococcus PCR on the Biofire meningitis/encephalitis panel((R)). Clin Microbiol Infect 24: 791–792. [DOI] [PubMed] [Google Scholar]
- 20.O’Halloran JA, Franklin A, Lainhart W, Burnham CA, Powderly W, Dubberke E, 2017. Pitfalls Associated With the Use of Molecular Diagnostic Panels in the Diagnosis of Cryptococcal Meningitis. Open Forum Infect Dis 4: ofx242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aberg JA, Watson J, Segal M, Chang LW, 2000. Clinical utility of monitoring serum cryptococcal antigen (sCRAG) titers in patients with AIDS-related cryptococcal disease. HIV Clin Trials 1: 1–6. [DOI] [PubMed] [Google Scholar]
- 22.Abassi M, Boulware DR, Rhein J, 2015. Cryptococcal Meningitis: Diagnosis and Management Update. Curr Trop Med Rep 2: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


