Abstract
The current review evaluates the potential of cannabidiol (CBD) as a promising pharmacotherapy for social anxiety disorder (SAD). Although a number of evidence-based treatments for SAD are available, less than a third of affected individuals experience symptom remission after one year of treatment. Therefore, improved treatment options are urgently needed, and CBD is one candidate medication that may have certain benefits over current pharmacotherapies, including the absence of sedating side effects, reduced abuse liability, and rapid course of action. The current review provides a brief overview of CBD’s mechanisms of action, neuroimaging in SAD, and evidence for CBD’s effects on the neural substrates of SAD, as well as systematically reviewing literature directly examining the efficacy of CBD for improving social anxiety among healthy volunteers and individuals with SAD. In both populations, acute CBD administration significantly decreased anxiety without co-occurring sedation. A single study has also shown chronic administration to decrease social anxiety symptoms in individuals with SAD. Collectively, the current literature suggests CBD may be a promising treatment for SAD. However, further research is needed to establish optimal dosing, assess the timecourse of CBD’s anxiolytic effects, evaluate long-term CBD administration, and explore sex differences in CBD for social anxiety.
Keywords: CBD, Efficacy, Side effects, Dosing, Public speaking
1. Introduction
Social Anxiety Disorder (SAD) is characterized by clinically significant fear of one or more specific social situations in which an individual may feel scrutinized, judged, or humiliated (American Psychiatric Association [APA], 2013). Critically, the anxiety related to these events also manifests in anticipatory anxiety and post-event processing: worry or dread surrounding the upcoming situation, even far beforehand, and rumination over their performance afterwards (APA, 2013; Clark and Wells, 1995). Consequently, individuals with SAD often develop avoidance behaviors to mitigate their discomfort (APA, 2013), leading them to appear shy or socially withdrawn, which can be mistaken as disinterest by others (Clark and Wells, 1995). This creates a cycle of distress in which the individual is consistently perceiving themselves as inferior and engaging in maladaptive coping strategies to avoid anxiety-inducing social situations (Rachman et al., 2000).
The lifetime prevalence of SAD in the United States is 7% annually; it is nearly twice as common among women and has a median onset age of 13 years (APA, 2013). SAD is associated with decreased quality of life, lower socioeconomic status, and reduced vocational productivity (APA, 2013). Despite the persistent impairments in social functioning that impact other aspects of life, only 50% of individuals seek treatment after experiencing symptoms for 15–20 years (APA, 2013). Of those who pursue clinical care, only 30% of individuals experience symptom remission within one year (APA, 2013). Therefore, improved access to effective treatment is imperative.
Several treatment options already exist. Cognitive-behavioral therapy (CBT) is a particularly effective psychosocial intervention for the treatment of SAD (Goldin et al., 2014; Nordahl et al., 2016; Rodebaugh et al., 2004), and first-line pharmacotherapies include selective serotonin reuptake inhibitors (SSRI) and selective norepinephrine reuptake inhibitors (SNRI) (Blanco et al., 2002; Garakani et al., 2020; National Collaborating Centre for Mental Health [NCCMH], 2013). While tricyclic antidepressants (TCA) and monoamine oxidase inhibitors (MAOI) remain in use as second-line options, the newer SSRIs and SNRIs have increased tolerability and fewer contraindications (Blanco et al., 2002; Garakani et al., 2020; NCCMH, 2013). Benzodiazepines are also still widely used and act much more rapidly (minutes versus weeks) but can lead to tolerance, abuse, and dependence, in addition to causing sedation and cognitive and motor impairments. Therefore, they are preferred for adjunctive short-term use with SSRIs, SNRIs, and/or psychosocial treatment (Balon and Starcevic, 2020; Garakani et al., 2020; Stewart, 2005). Atypical antipsychotics and beta-blockers have also been used off-label to augment antidepressants, regulating mood and autonomic activity; the latter may benefit individuals with SAD in performance situations as they are often hyper-aware of their bodily states (Davidson, 2006; Katzman et al., 2014). Overall, although there are a variety of existing treatment options for SAD, the large majority of affected individuals do not experience remission within one year (APA, 2013). Therefore, there is an urgent need for novel pharmacotherapeutic options to improve SAD treatment outcomes.
Emerging evidence suggests that cannabidiol (CBD), a phytocannabinoid in the cannabis plant, may be a promising treatment option for individuals with SAD. CBD is a non-intoxicating cannabinoid that has low abuse potential and considerable tolerability across a wide range of doses (Bergamaschi et al., 2011b; Larsen and Shahinas, 2020). Preclinical studies have demonstrated anxiolytic effects of CBD in models of SAD and suggest that CBD acts on various neural regions implicated in the disorder (Campos et al., 2013; Campos and Guimaraes, 2009; Gomes et al., 2011). Notably, CBD may have some key advantages over existing treatments; preliminary evidence suggests it can improve acute anxiety without psychoactive or sedative side effects, while chronic administration may have enduring benefits for improving symptoms over time (Bergamaschi et al., 2011b). Therefore, the current review will synthesize emerging evidence for CBD’s efficacy in ameliorating symptoms of social anxiety and highlight remaining gaps in the literature to pave the way for future evidence-based work in this area. To this end, we will first review CBD’s diverse mechanisms of action, human neuroimaging findings in SAD, and neuroimaging literature suggesting that CBD alters the function of neural regions implicated in SAD. Finally, we will systematically review existing studies that directly assess the effect of CBD administration on social anxiety symptoms in healthy volunteers and individuals with SAD.
1.1. CBD’s mechanisms of action
CBD has complex mechanisms of action, involving cannabinoid receptor (CB) type 1 and CB type 2 (Thomas et al., 2007), and G protein-coupled receptor 55 (GPR55) (Ryberg et al., 2007); it has further been demonstrated to be an agonist at the serotonin 1 A (5-HT1A) receptor (Russo et al., 2005) and the transient receptor potential vanilloid 1 (TRPV1) receptor (Bisogno et al., 2001). These receptors are densely expressed in the regions implicated in the neurobiology of SAD (Akimova et al., 2009; Herkenham et al., 1990; Tóth et al., 2005; reviewed below), suggesting that CBD administration may meaningfully modulate the function of key regions implicated in SAD pathophysiology. CBD’s effects on anxiety have been shown to exhibit an inverted U-shaped dose-response curve, with high doses potentially being ineffective due to its activation of TRPV1 receptors, which facilitates glutamate release inhibiting the anxiolytic effects of CBD modulated by 5-HT1A neurotransmission (Campos et al., 2013; Campos and Guimaraes, 2009; Gomes et al., 2011). This hypothesis is supported by preclinical evidence that the U-shaped dose-response curve is abolished when CBD administration is preceded by a TRPV1 antagonist (Campos and Guimaraes, 2009).
There is also emerging evidence implicating the presynaptic serotonergic system as being overactive in SAD, with increased serotonin synthesis and transporter availability in the anterior cingulate cortex (ACC) (Frick et al., 2015; Furmark et al., 2016), striatum (Frick et al., 2015; Hjorth et al., 2019), and amygdala (Frick et al., 2015; Furmark et al., 2016; Hjorth et al., 2019). During a public speaking task, SSRI treatment decreased amygdala serotonin synthesis rate and was associated with reduced symptoms and amygdala regional cerebral blood flow (rCBF) (Frick et al., 2016). As CBD appears to exert its anxiolytic effects through a 5-HT1A dependent mechanism (Campos et al., 2013; Gomes et al., 2011), this may represent a key mechanism supporting the efficacy of CBD for SAD.
1.2. Neuroimaging in SAD
Neuroimaging offers a non-invasive approach for assessing distinct patterns of neural functioning in vivo (Brammer, 2009) and a number of studies have applied neuroimaging methods to examine alterations in brain structure and regional activation among individuals with SAD. Neuroimaging studies that investigate the neural correlates of SAD most consistently find hyperactivity in the ACC (Amir et al., 2005; Klumpp et al., 2014; Onoda et al., 2010), amygdala (Blair et al., 2008b; Phan et al., 2006; Richey et al., 2016), hippocampus (Furmark et al., 2002; Stein et al., 2002), and insula (Boehme et al., 2014; Gentili et al., 2008; Klumpp et al., 2012; Straube et al., 2004; Terasawa et al., 2013; Wang et al., 2019), hypoactivity of the striatum (Becker et al., 2017; Levita et al., 2012; Sareen et al., 2007), as well as dysregulated prefrontal cortex (PFC) activation (Blair et al., 2008a; Guyer et al., 2008). Congruently, studies examining resting-state FC have also found altered FC among individuals with SAD, namely in the ACC and PFC (Ding et al., 2011; Liao et al., 2010; Manning et al., 2015). The structural literature has yielded less consistent findings but provides preliminary evidence for decreased gray matter volume in the right ITG (Liao et al., 2011; Syal et al., 2012), with changes in cortical thickness in several prefrontal areas (Frick et al., 2013; Gentili et al., 2008).
The ACC is strongly implicated in emotional regulation and cognitive control (Ray and Zald, 2012), and hyperactivity of the ACC may contribute to a tendency for individuals with SAD to quickly appraise negative stimuli and misinterpret neutral cues. Amygdala hyperactivity may represent another neural mechanism underlying heightened salience of negative social cues in this population. Similarly, relevant prefrontal regions are likely involved in the cognitive appraisal of social situations and attention towards salient stimuli (Kim et al., 2018; Prater et al., 2013; Tillfors et al., 2002). Greater insular activation in SAD is posited to relate to the misperception of one’s bodily arousal and surroundings (Straube et al., 2004), whereas ventral striatal dysfunction likely parallels avoidance behaviors and the inability to manage potentially negative evaluations (Schneier et al., 2009).
Overall, extant findings converge in suggesting neural activity is altered in individuals with SAD within the amygdala, insula, ACC, ventral striatum, and prefrontal regions. We next review neuroimaging literature in which CBD administration in healthy individuals has been observed to act on SAD-relevant regions during a variety of experimental paradigms.
1.3. Effects of CBD administration on neural substrates of SAD
Further evidence that CBD may be an effective treatment option for SAD comes from fMRI studies of healthy individuals demonstrating that CBD appears to act on the same neural regions where functional activation is altered in SAD: the ACC, PFC, insula, amygdala, hippocampus, and striatum. Therefore, we provide a brief review of neuroimaging literature examining the effects of CBD on SAD-relevant brain regions among healthy volunteers prior to systematically reviewing the preliminary literature directly examining the effects of CBD administration on social anxiety symptoms among in healthy volunteers undergoing social anxiety-inducing tasks and individuals with SAD.
1.3.1. Cortical findings
Extant literature in healthy volunteers has demonstrated that CBD administration alters functional responses of the PFC, insula, and ACC, using a variety of fMRI paradigms. Using response inhibition and fearful face tasks, CBD administration has been associated with changes in activation in both the medial PFC (mPFC) and lateral PFC, relative to placebo (Bhattacharyya et al., 2010), while studies using visual detection and verbal learning tasks demonstrated downregulated activation in the IFG (Bhattacharyya et al., 2012; O’Neill et al., 2021), mPFC, OFC, middle frontal gyrus (MFG), and superior frontal gyrus (Bhattacharyya et al., 2012). During response inhibition and auditory stimulation tasks, CBD has also been associated with reduced activation in regions of the insula (Borgwardt et al., 2008; Winton-Brown et al., 2011), whereas enhanced insula activation was found during a visual detection paradigm (Bhattacharyya et al., 2012). In an emotional processing task viewing fearful faces, acute CBD administration attenuated activation in the ACC (Fusar-Poli et al., 2009) and was further found to decrease FC from the ACC to the amygdala, correlating with diminished anxiety symptoms and autonomic arousal (Fusar-Poli et al., 2010). Effectively disrupting ACC-amygdala FC may aid in modulating the overactive emotional processing that is present in SAD (Fusar-Poli et al., 2010).
1.3.2. Subcortical findings
There is also evidence that CBD administration alters amygdalar, hippocampal, and striatal function during various tasks. Acute CBD administration decreased amygdalar and hippocampal activation during rest (Crippa et al., 2004) and during a fearful faces task (Bhattacharyya et al., 2010; Davies et al., 2020; Fusar-Poli et al., 2009). During response inhibition and verbal memory tasks, CBD increased activation in the striatum (Bhattacharyya et al., 2010); increased dorsal striatal FC during an oddball salience processing task was also found with the left caudate and left IFG, while reduced FC was seen with the left ACC and left MFG (Bhattacharyya et al., 2015).
Overall, acute CBD administration appears to downregulate activity in areas of the PFC, insula, ACC, amygdala, and hippocampus, while upregulating activity in the striatum. Based on the literature on the underlying neurobiology of SAD, these data suggest that acute CBD administration may effectively attenuate social anxiety symptoms. Accordingly, we next sought to systematically review studies that have focused specifically on measuring the effects of CBD administration on social anxiety.
2. Methods
A systematic literature search of Medline, Embase, and Psycinfo was conducted on June 25th, 2021, with 6 search terms encompassing the following parameters: social anxiety disorder (OR social anxiety, social phobia, sad) and cannabidiol (OR cbd). This produced 78 studies, and three additional studies were identified from other sources (see Fig. 1 for PRISMA flowchart). Studies were included in the current review if they utilized original data examining the effects of CBD administration on social anxiety symptoms. Studies were excluded if they a) did not include original data (i.e., conference abstract only, review papers), or b) did not assess social anxiety symptoms. Additionally, included studies were assessed for risk of bias using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) (Sterne et al., 2019).
Fig. 1.
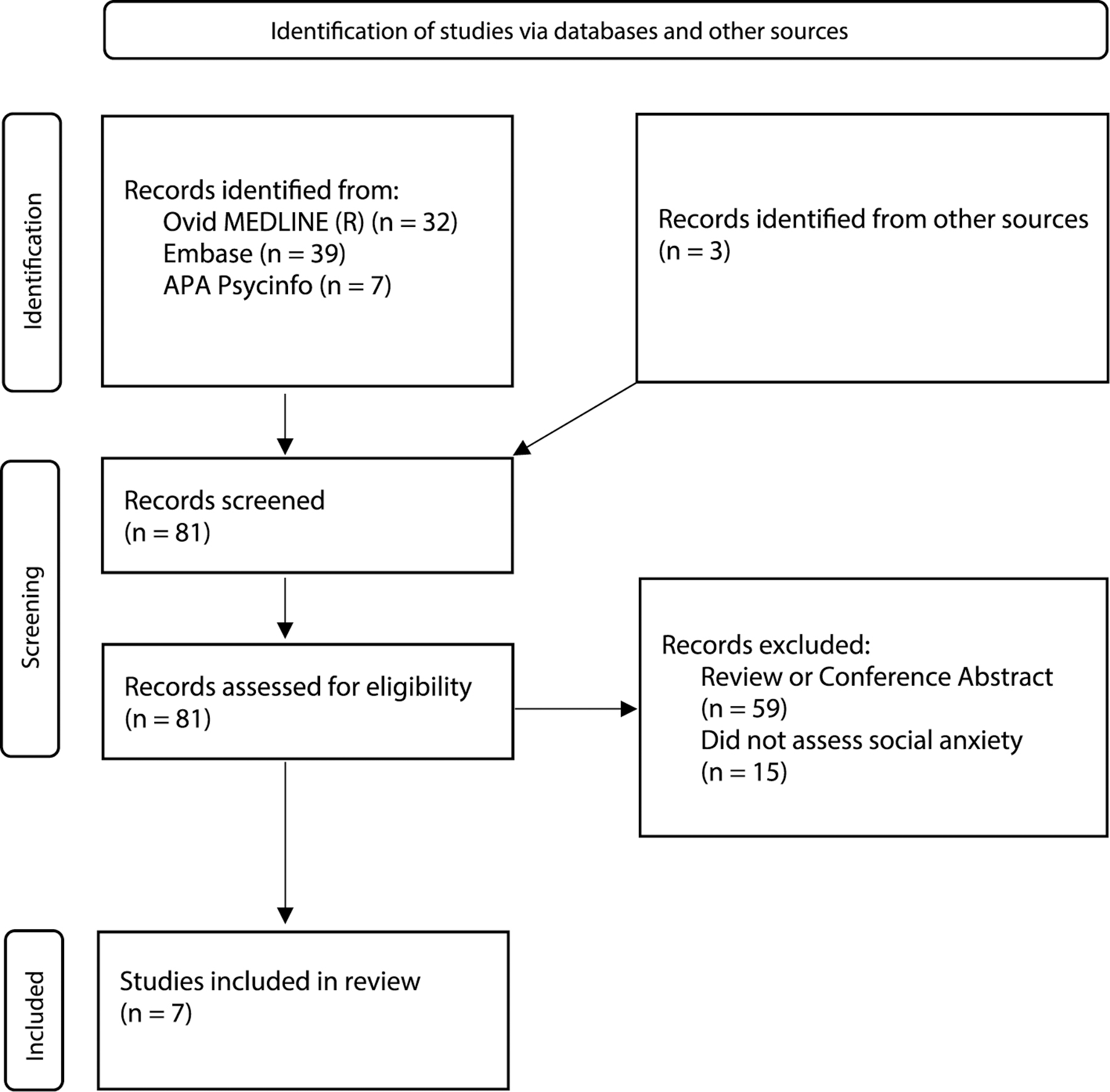
PRISMA Flowchart. Overview of the study selection process.
3. Results
Seven studies were identified for inclusion (see Fig. 1 for PRISMA flowchart), including 4 studies examining the effects of CBD on experimentally-induced social anxiety among healthy volunteers (summarized in Table 1A) and 3 studies assessing the impact of CBD on social anxiety symptoms among individuals with SAD (summarized in Table 1B). Together, these studies include data from n = 278 individuals (n = 71 individuals with SAD; n = 207 healthy volunteers). Notably, female participants were underrepresented (n = 100 female participants vs. n = 178 male participants), despite data that SAD is twice as prevalent among women. The bias assessment identified 5 articles with some concerns of bias (Bergamaschi et al., 2011a; Crippa et al., 2011; Linares et al., 2019; Zuardi et al., 1993, 2017) and 2 articles with a high risk of bias (Arndt and de Wit, 2017; Masataka, 2019) (see Tables 1A and 1B).
Table 1.
Studies examining the effect of CBD administration on social anxiety.
| Citation | Sample Gender | Sample Size | Primary Treatment | Interval Between CBD Admin and Testing | Experimental Paradigm | Primary Outcome Measure | Main Results (CBD v. placebo) | Additional Results | Side Effects Assessment | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Table 1A. Healthy Volunteer Literature. | ||||||||||
| Zuardi et al. (1993) | F/M | n = 40 (22 F, 18 M); n = 10 per treatment + placebo | 300mg CBD; 5mg Ipsapirone, 10mg Diazepam | 120min | Public Speaking Paradigm | VAMS | 300mg CBD reduced anxiety post-stress | Diazepam reduced anxiety pre- and post-stress, Ipsapirone reduced anxiety during the speech | Sedation - NS (Diazepam was associated with significant sedation, physical impairment) | Some concerns |
| Zuardi et al. (2017) | F/M | n = 59 (30 F, 29 M); n = 12 per 100 and 900mg CBD, Clonazepam, and placebo, n = 11 300mg CBD | 100, 300, 900mg CBD; 1mg Clonazepam | 160min | Public Speaking Paradigm | VAMS | 300mg CBD reduced anxiety post-stress | 1mg Clonazepam reduced anxiety during and post-stress | Sedation - NS (Clonazepam was associated with significant sedation) | Some concerns |
| Linares et al. (2019) | M | n = 57; n = 15 150mg, n = 15 300mg, n = 12 600mg, n = 15 placebo | 150, 300, 600mg CBD | 90min | Public Speaking Paradigm | VAMS | 300mg CBD reduced anxiety during stress | N/A | Sedation - NS | Some concerns |
| Arndt and de Wit, 2017 | F/M | n = 38 (19 F, 19 M); crossover design, 3 doses + placebo) | 300, 600, 900mg CBD | 160min | Emotional Stroop, IAPS, DEIT, ABT, Cyberball | POMS | CBD did not reduce anxiety | N/A | Sedation - NS | High |
|
Table 1B. Studies Including Individuals with Social Anxiety Disorder. | ||||||||||
| Bergamaschi et al. (2011) | M | n = 36; n = 12 CBD, n = 12 placebo, n = 12 HC no treatment | 600mg CBD | 90min | Public Speaking Paradigm | VAMS, SSPS-N | 600mg CBD reduced anxiety during stress | CBD SAD group had comparable self-evaluations to HC; SAD-placebo group had more negative | Sedation - NS | Some concerns |
| Crippa et al. (2011) | M | n = 10 (CBD + placebo; crossover design) | 400mg CBD | 110min | Single-photon emission computed tomography scan | VAMS, rCBF | 400mg CBD reduced anxiety pre-stress, adaptation, post-stress | rCBF ↓ in left parahippocampal-hippocampal region; rCBF ↑ in right PCC | Sedation - NS | Some concerns |
| Masataka, 2019 | F/M | n = 37 (11 F, 26 M; all participants had comorbid avoidant personality disorder); n = 17 CBD, n = 20 placebo | Chronic CBD admin (300mg CBD daily for 1 month) | N/A | N/A | FNE, LSAS | 300mg CBD reduced anxiety | CBD group more likely to seek further treatment | Not systematically evaluated; no health complaints reported | High |
Note. CBD = cannabidiol, SAD = social anxiety disorder, HC = healthy control, NS = not seen, IAPS = International Affective Picture System, DEIT = Dynamic Emotion Identification Task, ABT = Attentional Bias Task, rCBF = regional cerebral blood flow, PCC = posterior cingulate cortex, VAMS = Visual Analog Mood Scale, POMS = Profile of Mood States Questionnaire, SSPS-N = Self-Statements During Public Speaking Scale, FNE = Fear of Negative Evaluation Questionnaire, LSAS = Liebowitz Social Anxiety Scale.
3.1. Effects of CBD administration on experimentally-induced social anxiety in healthy volunteers
Published studies provide evidence for an anxiolytic effect of CBD in paradigms where social anxiety-like symptoms are elicited in healthy volunteers via experimental public speaking paradigms (Linares et al., 2019; Zuardi et al., 1993, 2017). Zuardi et al. (1993) conducted a pilot study investigating the anxiolytic effects of CBD (300mg) versus placebo, a benzodiazepine, and a partial serotonergic agonist. CBD (300mg) significantly decreased post-stress anxiety relative to placebo, whereas benzodiazepine treatment considerably reduced ratings of anxiety both during the speech and post-stress. The post-stress timepoint may be particularly critical for individuals with SAD, given literature that these individuals display characteristic patterns of post-event processing, scrutinizing their prior social performance (Clark and Wells, 1995; Rachman et al., 2000; Zuardi et al., 1993). While healthy volunteers may not experience post-stress anxiety to the same extent as individuals with SAD, the placebo group did display significant elevations in post-stress anxiety relative to baseline (Rachman et al., 2000).
Notably, benzodiazepine treatment was also associated with significant increases in sedation, motor discoordination, and dizziness at various timepoints during the public speaking paradigm, as well as showing a trend towards elevations in cognitive impairment throughout. CBD did not cause these same deficits, suggesting that CBD may effectively attenuate social anxiety symptoms without inducing impairments in cognition and motor functioning.
To assess a wider range of CBD doses, Zuardi et al. (2017) conducted a follow-up study assessing the anxiolytic effects of 100mg, 300mg, and 900mg doses of CBD, compared to placebo and benzodiazepine treatment. Similar to the pilot study, individuals in the 300mg CBD group showed significantly reduced anxiety during the speech compared to individuals in the 900mg CBD group, as well as significant reductions in post-stress anxiety relative to individuals in the 100mg CBD and placebo groups. Again, individuals in the benzodiazepine group reported significant reductions in anxiety relative to placebo both during the speech and post-stress, as well as increased post-stress sedation. These results align with the pilot study in supporting the efficacy of 300mg CBD for attenuating anxiety without concomitant sedation and demonstrate evidence for an inverted U-shaped dose-response curve in humans, congruent with prior animal studies (Guimaraes et al., 1990; Levin et al., 2014; Nazario et al., 2015; Zuardi et al., 2017).
Further, Linares et al. (2019) investigated the anxiolytic effects of CBD (150, 300, 600mg) versus placebo using a public speaking task. Individuals in the 300mg CBD group experienced significantly reduced anxiety during the speech relative to placebo, whereas individuals in the 150 and 600mg groups exhibited minor reductions in anxiety throughout the study compared to placebo, consistent with the non-significant effect of the low and high CBD doses observed in the previous study.
Congruent with Zuardi et al. (2017), Linares et al. (2019) found additional evidence for an anxiolytic effect of 300mg CBD and an inverted U-shaped dose-response curve. A sedative effect of CBD was again not detected, providing additional evidence that CBD may have an advantage over benzodiazepines in the cognitive and physical impairment domains. Overall, these studies suggest 300mg CBD appears to be the most effective anxiolytic dose (Linares et al., 2019; Zuardi et al., 1993, 2017).
While the aforementioned studies examined the anxiolytic effects of CBD in public speaking situations, Arndt and de Wit (2017) explored the effects of CBD (300, 600, and 900mg) on responses to emotional stimuli. Behavioral tasks involved emotional arousal, social rejection, and sensitivity and attentional bias in evaluating facial expressions. In contrast to the other studies reviewed, no significant effects were found for any of the CBD doses relative to placebo. Again, individuals treated with CBD did not experience any subjective drug effects, unlike benzodiazepines (Arndt and de Wit, 2017; Linares et al., 2019; Zuardi et al., 1993; Zuardi et al., 2017).
3.2. Effects of CBD administration on social anxiety symptoms in patients with SAD
Although a fear of public speaking is present in a portion of the general population, performing, being observed, and the potential for negative evaluation can cause substantial dread and avoidance among individuals with SAD (Furmark et al., 1999; Heimberg et al., 2014; Stein et al., 1994). Consequently, Bergamaschi et al. (2011a) used an experimental public speaking paradigm to investigate the effects of CBD (600mg) versus placebo among individuals with SAD. Healthy controls (HC) also completed the task but did not receive treatment.
Individuals in the CBD group exhibited significantly reduced anxiety relative to the placebo group during the speech, though their anxiety remained higher than HC. Individuals with SAD who received placebo had markedly more negative self-evaluations of their public speaking performance than the CBD and HC groups. Individuals with SAD who received CBD reported more alertness than the HC group, which likely reflects greater anxiety induced by the paradigm. Therefore, these findings extend the healthy control literature in finding a significant anxiolytic effect of CBD among individuals with SAD, albeit at a higher CBD dose.
Crippa et al. (2011) assessed the neural correlates of the anxiolytic effects of acute CBD (400mg) administration in treatment-naïve participants with SAD. Single photon emission computed tomography (SPECT) was used to measure resting rCBF and was deemed anxiety-inducing due to its novelty to participants. The medial temporal cortex (parahippocampal gyrus, amygdala, and hippocampus) and posterior cingulate cortex (PCC) were selected as a priori regions-of-interest.
Individuals in the CBD condition reported significantly attenuated anxiety relative to placebo. They also exhibited significantly decreased rCBF in the left parahippocampal gyrus and hippocampus, and increased rCBF in the right PCC. Elevated activity in a parahippocampal-hippocampal region has also been observed among individuals with SAD during an anxiogenic task and was ameliorated by SSRI treatment (Furmark et al., 2002); the present results suggest CBD may also reduce anxiety via a similar mechanism. Further, the authors also report a lack of sedation in the CBD condition, underscoring previous findings in healthy volunteers and individuals with SAD (Bergamaschi et al., 2011a; Linares et al., 2019; Zuardi et al., 1993, 2017).
Finally, Masataka (2019) investigated the anxiolytic effects of repeated CBD (300mg) administration versus placebo in treatment-naïve teenagers with SAD and comorbid avoidant personality disorder. During a one-month intervention, a clinical psychologist made daily visits to the home of each participant to orally administer the treatment. The groups did not differ in their level of social anxiety symptoms prior to treatment, and the CBD group (300mg) demonstrated significant symptom reduction across the intervention period.
These results suggest that repeated administration of CBD is effective in decreasing social anxiety symptoms, consistent with prior studies examining acute CBD administration prior to anxiogenic experimental public speaking paradigms (Bergamaschi et al., 2011a; Linares et al., 2019; Zuardi et al., 1993, 2017). As levels of social anxiety were not systematically evaluated throughout the intervention period, the timecourse of symptom improvements remains unknown. Future studies involving chronic CBD administration are needed to assess whether symptom improvements occur rapidly or gradually over the course of sustained treatment.
4. Discussion
SAD is a debilitating anxiety disorder characterized by persistent and intense fear of specific social situations leading to compensatory avoidance behaviors, which is associated with substantially decreased quality of life. While a variety of evidence-based treatment options exist, a minority of patients experience symptom remission following 1-year of treatment, and existing pharmacotherapies are limited by a delayed course of action for SSRIs/SNRIs, and high abuse liability and problematic side effects in the case of benzodiazepines. CBD has emerged a promising treatment candidate for SAD and preliminary neuroimaging data suggests that CBD acts on the same brain regions known to be altered in SAD. Additionally, CBD may have key advantages over existing treatments, including a rapid course of action, reduced abuse liability and potential for drug interactions with alcohol and opioids, as well as reduced sedative and cognitive side effects. However, few studies to date have directly examined the efficacy of CBD for SAD. Therefore, the current systematic review examined the preliminary extant literature addressing the efficacy of CBD for SAD in human participants in order to synthesize current findings and highlight important areas for future research.
Overall, the literature reviewed supports the anxiolytic effect of CBD administration, both among healthy volunteers undergoing experimental social anxiety paradigms, as well as individuals with SAD. Within healthy volunteers, a reduction in anxiety was observed in experimental public speaking tasks (Zuardi et al., 1993) in an inverted U-shaped dose-response curve (Linares et al., 2019; Zuardi et al., 2017). Notably, significant anxiolytic effects of CBD occurred without the concomitant sedation observed with benzodiazepine treatment (Linares et al., 2019; Zuardi et al., 1993, 2017). In individuals with SAD, social anxiety symptoms were ameliorated by CBD administration in a public speaking paradigm (Bergamaschi et al., 2011a), a SPECT scan (Crippa et al., 2011), and a longitudinal study involving one month of daily CBD treatment (Masataka, 2019). Again, studies including SAD patients also observed significant improvements in anxiety without cognitive or sedative side effects (Bergamaschi et al., 2011a; Crippa et al., 2011).
With regard to the magnitude of CBD’s anxiolytic effects, studies in healthy volunteers have reported that pre-stress subjective anxiety ratings were approximately 2–27% lower in the CBD condition versus placebo, ratings during the stress induction were approximately 10–12% lower, and post-stress rating were approximately 16–52% lower for CBD relative to placebo (Zuardi et al., 1993, 2017). Among individuals with SAD, studies involving acute CBD administration reported that pre-stress ratings were approximately 18–45% lower in the CBD condition relative to placebo, ratings were approximately 18–44% lower during stress induction, and 27–78% lower following the stressor (Bergamaschi et al., 2011a; Crippa et al., 2011). For the one study assessing chronic CBD administration, individuals in the CBD condition reported symptom reductions of approximately 25% and 16% (depending on the outcome measure) following one month of treatment, relative to 0% and 4%, respectively, for individuals in the placebo condition (Masataka, 2019).
Although systematic side effect assessments were not conducted in the studies reviewed here, data suggest that CBD administration is well-tolerated (Bergamaschi et al., 2011b; Taylor et al., 2018). A recent meta-analysis of CBD’s adverse effects (AEs) following 1–14 weeks of treatment for various indications reported sedation, decreased appetite, diarrhea, and somnolence as the most common side effects (Chesney et al., 2020). Notably, AEs were reported more frequently among individuals taking higher CBD doses and among individuals in treatment for epilepsy (Chesney et al., 2020), likely due to drug-drug interactions with antiepileptic drugs (Gaston et al., 2017; Geffrey et al., 2015; Morrison et al., 2019). In the non-epilepsy studies, solely diarrhea was more frequent with CBD treatment versus placebo (Chesney et al., 2020). Anxiolytic effects were observed with considerably lower, acute doses of CBD (Bergamaschi et al., 2011a; Crippa et al., 2011; Linares et al., 2019; Zuardi et al., 1993, 2017) relative to doses administered for other indications (Chesney et al., 2020), likely mitigating potential dose-related AEs. Nonetheless, it is essential that possible interactions with SSRIs/SNRIs and benzodiazepines are thoroughly assessed prior to CBD being used in the treatment of SAD.
The existing literature also provides important preliminary data to evaluate optimal CBD dosing. While administration of 100–900mg CBD was examined, extant studies suggest 300mg may be the most effective anxiolytic dose (Linares et al., 2019; Masataka, 2019; Zuardi et al., 1993, 2017), likely due to the inverted U-shaped dose-response curve identified in preclinical (Campos et al., 2013; Campos and Guimaraes, 2009; Gomes et al., 2011) and clinical (Linares et al., 2019; Zuardi et al., 2017) studies. Relative to placebo, 300mg CBD significantly attenuated anxiety both when administered acutely (Linares et al., 2019; Zuardi et al., 1993, 2017) and chronically (Masataka, 2019), among healthy volunteers (Zuardi et al., 1993, 2017) and individuals with SAD (Bergamaschi et al., 2011a). However, whereas several studies compared multiple CBD doses in healthy volunteers (Linares et al., 2019; Zuardi et al., 1993, 2017), existing studies including patient populations have only examined a single dose (600mg (Bergamaschi et al., 2011a), 400mg (Crippa et al., 2011), and 300mg (Masataka, 2019)). Therefore, future studies directly comparing different CBD doses among patients with SAD are urgently needed.
Collectively, these data suggest that CBD administration may have key benefits over existing pharmacotherapies for SAD (Blanco et al., 2002). In particular, CBD appears to reduce social anxiety symptoms quickly without impairing cognitive or physical functioning. The rapid course of action can present an advantage over the several-week-long latency period of SSRIs and SNRIs, and the lack of functionally impairing side effects is a notable advantage over benzodiazepines. Moreover, unlike benzodiazepines, CBD has a low abuse potential and lacks the potential for fatal interactions with alcohol and opioids (Bergamaschi et al., 2011b; Jones et al., 2014; Stewart, 2005; Tori et al., 2020). Since CBD is not associated with these risks, it may be especially appealing to individuals with SAD that are younger, older, or those with comorbid substance use disorders.
While the majority of studies were judged to have some concerns with regard to their overall risk of bias, this was largely driven by the fact that studies did not include a preregistered analysis plan. Risk of bias across other domains (randomization process, deviations from intended interventions, missing outcome data, and measurement of the outcome, as well as a sixth domain regarding crossover effects, for crossover studies only) was assessed to be low in all but two studies. Masataka (2019) was deemed high risk with regard to missing outcome data that disproportionately affected the experimental group, where three participants dropped out due to an adverse taste associated with the CBD solution (see Supplement S1). Additionally, the Arndt and de Wit (2017) study was delineated as high risk, having an insufficient washout period (1 week) to mitigate crossover effects of the tasks across trials. Therefore, the majority of studies had a low risk of bias across most domains, whereas Masataka (2019) and Arndt and de Wit (2017) were found to have potential bias attributed to missing outcome data and crossover effects, respectively (see Supplement S1).
Although the studies converged in observing acute anxiolytic effects of CBD administration, there was substantial variability in the timing between CBD administration and testing, ranging from 90 to 160 min (Bergamaschi et al., 2011a; Crippa et al., 2011; Linares et al., 2019; Zuardi et al., 1993, 2017). In experimental public speaking paradigms, differences in this span may have led to significantly reduced anxiety relative to placebo during the speech (Bergamaschi et al., 2011a; Linares et al., 2019) versus post-speech (Zuardi et al., 1993, 2017) phases of the task. Individuals with SAD experience elevated social anxiety symptoms during a stressor, as well as persistent post-stress anxiety due to negatively biased post-event processing (Clark and Wells, 1995; Rachman et al., 2000). Several studies reviewed demonstrated that CBD ameliorated post-stress anxiety (Crippa et al., 2011; Zuardi et al., 1993, 2017) and negative self-evaluations (Bergamaschi et al., 2011a). Therefore, it has the potential to improve anxiety symptoms both during and following stressful events, aiding in diminishing rumination and maladaptive avoidance behaviors. However, it is essential that future studies systematically evaluate the optimal timing of administration and duration of CBD’s anxiolytic effects, as well as assessing varying doses in individuals with SAD.
In contrast to the majority of studies reviewed, Arndt and de Wit (2017) did not detect anxiolytic effects of CBD (300, 600, 900mg). The discrepancy in these results may be attributable to practice effects from repeated task administration, potential tolerance to CBD, or reduced CBD exposure due to drug administration when participants were fasted. Participants in the current study completed each task on four different occasions one week apart, which may have led the tasks to become less salient over time. Additionally, participants in this study had a much higher level of current cannabis use relative to subjects in the other studies reviewed. Specifically, 14 participants in the current study reported using cannabis an average of five times per month. Although it is impossible to know the CBD content of the cannabis used, this raises the possibility that these participants could have some level of tolerance to CBD exposure. Finally, participants agreed to fast prior to CBD administration (although they were given a granola bar at baseline), which may have limited their CBD exposure, as pharmacokinetic analyses have shown significantly greater CBD absorption in fed versus fasted states (Taylor et al., 2018). Future studies should assess whether CBD attenuates anxiety on tasks aside from public speaking paradigms among individuals with SAD to evaluate the specificity of CBD’s anxiolytic effects in this patient population.
While the majority of the reviewed literature investigated acute CBD administration (Arndt and de Wit, 2017; Bergamaschi et al., 2011a; Crippa et al., 2011; Linares et al., 2019; Zuardi et al., 1993; Zuardi et al., 2017), only one chronic administration study in individuals with SAD has been undertaken (Masataka, 2019). Over one month, 300mg CBD was given daily to participants and markedly reduced their social anxiety symptoms relative to placebo. Although the authors did not systematically evaluate cognitive or sedative side effects, no adverse effects were reported during or after the intervention (Masataka, 2019). Furthermore, the CBD group was significantly more likely to seek further treatment (medication and CBT) than the placebo group (Masataka, 2019), suggesting that chronic CBD administration can aid in removing barriers to additional treatment by decreasing symptoms. More chronic administration studies in individuals with SAD are thus needed to support these findings and carefully investigate potential side effects of long-term use.
Although nearly twice as many women than men are diagnosed with SAD (APA, 2013), women have been underrepresented in the current literature, and no studies have examined sex differences in the efficacy of CBD for treating social anxiety. This is particularly essential given pharmacokinetic data suggesting that women receive greater exposure from the same dose of CBD (Nadulski et al., 2005), and known sex differences in the effects of existing pharmacotherapies for SAD. For example, benzodiazepines are prescribed twice as often to women (Olfson et al., 2015), despite data suggesting women experience more adverse effects than men (Zucker and Prendergast, 2020) and concerning their abuse potential (Stewart, 2005). Therefore, it is critical that future studies include both male and female participants with SAD and assess sex differences in the efficacy, dosing, and side effect profile of CBD administration.
Overall, the preliminary extant human literature supports the potential efficacy of CBD for social anxiety. Existing data suggest that acute administration of CBD significantly attenuates social anxiety, without significant sedation or cognitive impairment. However, additional research is needed to determine optimal dosing among individuals with SAD, assess the timecourse of CBD’s acute effects, examine the efficacy and side effect profile of chronic CBD administration, assess sex differences in the use of CBD for social anxiety, as well as investigate CBD’s mechanisms of action.
Supplementary Material
Acknowledgements
We thank Dr. Sarah W. Yip for providing feedback on the manuscript.
Funding
This work was supported by the National Institute of Drug Abuse (NIDA) K08DA051667 and Women’s Health Research at Yale (WHRY) #1084 to SL.
Footnotes
Conflict of interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.psycom.2022.100074.
References
- Akimova E, Lanzenberger R, Kasper S, 2009. The serotonin-1A receptor in anxiety disorders. Biol. Psychiatr 66 (7), 627–635. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Anxiety Disorders, Diagnostic and Statistical Manual of Mental Disorders, fifth ed., vol. 5. DSM-, Washington, D.C., pp. 202–208 [Google Scholar]
- Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS, 2005. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol. Psychiatr 57 (9), 975–981. [DOI] [PubMed] [Google Scholar]
- Arndt DL, de Wit H, 2017. Cannabidiol does not dampen responses to emotional stimuli in healthy adults. Cannabis Cannabinoid Res 2 (1), 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balon R, Starcevic V, 2020. Role of benzodiazepines in anxiety disorders. Adv. Exp. Med. Biol 1191, 367–388. [DOI] [PubMed] [Google Scholar]
- Becker MPI, Simon D, Miltner WHR, Straube T, 2017. Altered activation of the ventral striatum under performance-related observation in social anxiety disorder. Psychol. Med 47 (14), 2502–2512. [DOI] [PubMed] [Google Scholar]
- Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, Kapczinski F, Quevedo J, Roesler R, Schroder N, Nardi AE, Martin-Santos R, Hallak JE, Zuardi AW, Crippa JA, 2011a. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology 36 (6), 1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi MM, Queiroz RH, Zuardi AW, Crippa JA, 2011b. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr. Drug Saf 6 (4), 237–249. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Crippa JA, Allen P, Martin-Santos R, Borgwardt S, Fusar-Poli P, Rubia K, Kambeitz J, O’Carroll C, Seal ML, Giampietro V, Brammer M, Zuardi AW, Atakan Z, McGuire PK, 2012. Induction of psychosis by Delta9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch. Gen. Psychiatr 69 (1), 27–36. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Falkenberg I, Martin-Santos R, Atakan Z, Crippa JA, Giampietro V, Brammer M, McGuire P, 2015. Cannabinoid modulation of functional connectivity within regions processing attentional salience. Neuropsychopharmacology 40 (6), 1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, Nosarti C, Cm OC, Seal M, Allen P, Mehta MA, Stone JM, Tunstall N, Giampietro V, Kapur S, Murray RM, Zuardi AW, Crippa JA, Atakan Z, McGuire PK, 2010. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 35 (3), 764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V, 2001. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol 134 (4), 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K, Geraci M, Devido J, McCaffrey D, Chen G, Vythilingam M, Ng P, Hollon N, Jones M, Blair RJ, Pine DS, 2008a. Neural response to self- and other referential praise and criticism in generalized social phobia. Arch. Gen. Psychiatr 65 (10), 1176–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, McCaffrey D, Vythilingam M, Finger E, Mondillo K, Jacobs M, Charney DS, Blair RJ, Drevets WC, Pine DS, 2008b. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am. J. Psychiatr 165 (9), 1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, Antia SX, Liebowitz MR, 2002. Pharmacotherapy of social anxiety disorder. Biol. Psychiatr 51 (1), 109–120. [DOI] [PubMed] [Google Scholar]
- Boehme S, Ritter V, Tefikow S, Stangier U, Strauss B, Miltner WH, Straube T, 2014. Brain activation during anticipatory anxiety in social anxiety disorder. Soc. Cognit. Affect Neurosci 9 (9), 1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgwardt SJ, Allen P, Bhattacharyya S, Fusar-Poli P, Crippa JA, Seal ML, Fraccaro V, Atakan Z, Martin-Santos R, O’Carroll C, Rubia K, McGuire PK, 2008. Neural basis of Delta-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol. Psychiatr 64 (11), 966–973. [DOI] [PubMed] [Google Scholar]
- Brammer M, 2009. The role of neuroimaging in diagnosis and personalized medicine-current position and likely future directions. Dialogues Clin. Neurosci 11 (4), 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AC, de Paula Soares V, Carvalho MC, Ferreira FR, Vicente MA, Brandao ML, Zuardi AW, Zangrossi H Jr., Guimaraes FS, 2013. Involvement of serotonin-mediated neurotransmission in the dorsal periaqueductal gray matter on cannabidiol chronic effects in panic-like responses in rats. Psychopharmacology (Berl) 226 (1), 13–24. [DOI] [PubMed] [Google Scholar]
- Campos AC, Guimaraes FS, 2009. Evidence for a potential role for TRPV1 receptors in the dorsolateral periaqueductal gray in the attenuation of the anxiolytic effects of cannabinoids. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 33 (8), 1517–1521. [DOI] [PubMed] [Google Scholar]
- Chesney E, Oliver D, Green A, Sovi S, Wilson J, Englund A, Freeman TP, McGuire P, 2020. Adverse effects of cannabidiol: a systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology 45 (11), 1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DM, Wells A, 1995. A Cognitive Model of Social Phobia, Social Phobia: Diagnosis, Assessment, and Treatment The Guilford Press, New York, NY, US, pp. 69–93. [Google Scholar]
- Crippa JA, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FL, Martin-Santos R, Simoes MV, Bhattacharyya S, Fusar-Poli P, Atakan Z, Santos Filho A, Freitas-Ferrari MC, McGuire PK, Zuardi AW, Busatto GF, Hallak JE, 2011. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J. Psychopharmacol 25 (1), 121–130. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Zuardi AW, Garrido GE, Wichert-Ana L, Guarnieri R, Ferrari L, Azevedo-Marques PM, Hallak JE, McGuire PK, Filho Busatto G, 2004. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology 29 (2), 417–426. [DOI] [PubMed] [Google Scholar]
- Davidson JR, 2006. Pharmacotherapy of social anxiety disorder: what does the evidence tell us? J. Clin. Psychiatr 67 (Suppl. 12), 20–26. [PubMed] [Google Scholar]
- Davies C, Wilson R, Appiah-Kusi E, Blest-Hopley G, Brammer M, Perez J, Murray RM, Allen P, Bossong MG, McGuire P, Bhattacharyya S, 2020. A single dose of cannabidiol modulates medial temporal and striatal function during fear processing in people at clinical high risk for psychosis. Transl. Psychiatry 10 (1), 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Chen H, Qiu C, Liao W, Warwick JM, Duan X, Zhang W, Gong Q, 2011. Disrupted functional connectivity in social anxiety disorder: a resting-state fMRI study. Magn. Reson. Imaging 29 (5), 701–711. [DOI] [PubMed] [Google Scholar]
- Frick A, Ahs F, Appel L, Jonasson M, Wahlstedt K, Bani M, Merlo Pich E, Bettica P, Langstrom B, Lubberink M, Fredrikson M, Furmark T, 2016. Reduced serotonin synthesis and regional cerebral blood flow after anxiolytic treatment of social anxiety disorder. Eur. Neuropsychopharmacol 26 (11), 1775–1783. [DOI] [PubMed] [Google Scholar]
- Frick A, Ahs F, Engman J, Jonasson M, Alaie I, Bjorkstrand J, Frans O, Faria V, Linnman C, Appel L, Wahlstedt K, Lubberink M, Fredrikson M, Furmark T, 2015. Serotonin synthesis and reuptake in social anxiety disorder: a positron emission tomography study. JAMA Psychiatr 72 (8), 794–802. [DOI] [PubMed] [Google Scholar]
- Frick A, Howner K, Fischer H, Eskildsen SF, Kristiansson M, Furmark T, 2013. Cortical thickness alterations in social anxiety disorder. Neurosci. Lett 536, 52–55. [DOI] [PubMed] [Google Scholar]
- Furmark T, Marteinsdottir I, Frick A, Heurling K, Tillfors M, Appel L, Antoni G, Hartvig P, Fischer H, Langstrom B, Eriksson E, Fredrikson M, 2016. Serotonin synthesis rate and the tryptophan hydroxylase-2: G-703T polymorphism in social anxiety disorder. J. Psychopharmacol 30 (10), 1028–1035. [DOI] [PubMed] [Google Scholar]
- Furmark T, Tillfors M, Everz P, Marteinsdottir I, Gefvert O, Fredrikson M, 1999. Social phobia in the general population: prevalence and sociodemographic profile. Soc. Psychiatr. Psychiatr. Epidemiol 34 (8), 416–424. [DOI] [PubMed] [Google Scholar]
- Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, Fredrikson M, 2002. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Arch. Gen. Psychiatr 59 (5), 425–433. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, Borgwardt S, Martin-Santos R, Seal ML, O’Carrol C, Atakan Z, Zuardi AW, McGuire P, 2010. Modulation of effective connectivity during emotional processing by Delta 9-tetrahydrocannabinol and cannabidiol. Int. J. Neuropsychopharmacol 13 (4), 421–432. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, Seal M, Surguladze SA, O’Carrol C, Atakan Z, Zuardi AW, McGuire PK, 2009. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch. Gen. Psychiatr 66 (1), 95–105. [DOI] [PubMed] [Google Scholar]
- Garakani A, Murrough JW, Freire RC, Thom RP, Larkin K, Buono FD, Iosifescu DV, 2020. Pharmacotherapy of anxiety disorders: current and emerging treatment options. Front. Psychiatr 11, 595584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston TE, Bebin EM, Cutter GR, Liu Y, Szaflarski JP, Program UC, 2017. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia 58 (9), 1586–1592. [DOI] [PubMed] [Google Scholar]
- Geffrey AL, Pollack SF, Bruno PL, Thiele EA, 2015. Drug–drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 56 (8), 1246–1251. [DOI] [PubMed] [Google Scholar]
- Gentili C, Gobbini MI, Ricciardi E, Vanello N, Pietrini P, Haxby JV, Guazzelli M, 2008. Differential modulation of neural activity throughout the distributed neural system for face perception in patients with Social Phobia and healthy subjects. Brain Res. Bull 77 (5), 286–292. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Lee I, Ziv M, Jazaieri H, Heimberg RG, Gross JJ, 2014. Trajectories of change in emotion regulation and social anxiety during cognitive-behavioral therapy for social anxiety disorder. Behav. Res. Ther 56, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Resstel LB, Guimaraes FS, 2011. The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology (Berl) 213 (2–3), 465–473. [DOI] [PubMed] [Google Scholar]
- Guimaraes FS, Chiaretti TM, Graeff FG, Zuardi AW, 1990. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology (Berl) 100 (4), 558–559. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Chen G, Blair RJ, Leibenluft E, Fox NA, Ernst M, Pine DS, Nelson EE, 2008. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch. Gen. Psychiatr 65 (11), 1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg RG, Hofmann SG, Liebowitz MR, Schneier FR, Smits JA, Stein MB, Hinton DE, Craske MG, 2014. Social anxiety disorder in DSM-5. Depress. Anxiety 31 (6), 472–479. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, De Costa BR, Rice KC, 1990. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA 87 (5), 1932–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth OR, Frick A, Gingnell M, Hoppe JM, Faria V, Hultberg S, Alaie I, Mansson KNT, Wahlstedt K, Jonasson M, Lubberink M, Antoni G, Fredrikson M, Furmark T, 2019. Expression and co-expression of serotonin and dopamine transporters in social anxiety disorder: a multitracer positron emission tomography study. Mol. Psychiatr 26 (8), 3970–3979. [DOI] [PubMed] [Google Scholar]
- Jones CM, Paulozzi LJ, Mack KA, 2014. Centers for Disease, C., Prevention, 2014. Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb. Mortal. Wkly. Rep 63 (40), 881–885. [PMC free article] [PubMed] [Google Scholar]
- Katzman MA, Bleau P, Blier P, Chokka P, Kjernisted K, Van Ameringen M, Canadian Anxiety Guidelines Initiative Group on behalf of the Anxiety Disorders Association of Canada/Association Canadienne des troubles, a., McGill U, Antony MM, Bouchard S, Brunet A, Flament M, Grigoriadis S, Mendlowitz S, O’Connor K, Rabheru K, Richter PM, Robichaud M, Walker JR, 2014. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatr 14 Suppl. 1, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Shin JE, Lee YI, Kim H, Jo HJ, Choi SH, 2018. Neural evidence for persistent attentional bias to threats in patients with social anxiety disorder. Soc. Cognit. Affect Neurosci 13 (12), 1327–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Angstadt M, Phan KL, 2012. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol. Psychol 89 (1), 273–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Keutmann MK, Fitzgerald DA, Shankman SA, Phan KL, 2014. Resting state amygdala-prefrontal connectivity predicts symptom change after cognitive behavioral therapy in generalized social anxiety disorder. Biol. Mood Anxiety Disord 4 (1), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C, Shahinas J, 2020. Dosage, efficacy and safety of cannabidiol administration in adults: a systematic review of human trials. J. Clin. Med. Res 12 (3), 129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R, Peres FF, Almeida V, Calzavara MB, Zuardi AW, Hallak JE, Crippa JA, Abilio VC, 2014. Effects of cannabinoid drugs on the deficit of prepulse inhibition of startle in an animal model of schizophrenia: the SHR strain. Front. Pharmacol 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Hoskin R, Champi S, 2012. Avoidance of harm and anxiety: a role for the nucleus accumbens. Neuroimage 62 (1), 189–198. [DOI] [PubMed] [Google Scholar]
- Liao W, Chen H, Feng Y, Mantini D, Gentili C, Pan Z, Ding J, Duan X, Qiu C, Lui S, Gong Q, Zhang W, 2010. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage 52 (4), 1549–1558. [DOI] [PubMed] [Google Scholar]
- Liao W, Xu Q, Mantini D, Ding J, Machado-de-Sousa JP, Hallak JE, Trzesniak C, Qiu C, Zeng L, Zhang W, Crippa JA, Gong Q, Chen H, 2011. Altered gray matter morphometry and resting-state functional and structural connectivity in social anxiety disorder . Brain Res 1388, 167–177. [DOI] [PubMed] [Google Scholar]
- Linares IM, Zuardi AW, Pereira LC, Queiroz RH, Mechoulam R, Guimaraes FS, Crippa JA, 2019. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Br. J. Psychiatry 41 (1), 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning J, Reynolds G, Saygin ZM, Hofmann SG, Pollack M, Gabrieli JD, Whitfield-Gabrieli S, 2015. Altered resting-state functional connectivity of the frontal-striatal reward system in social anxiety disorder. PLoS One 10 (4), e0125286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masataka N, 2019. Anxiolytic effects of repeated cannabidiol treatment in teenagers with social anxiety disorders. Front. Psychol 10, 2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison G, Crockett J, Blakey G, Sommerville K, 2019. A phase 1, open-label, pharmacokinetic trial to investigate possible drug-drug interactions between clobazam, stiripentol, or valproate and cannabidiol in healthy subjects. Clinical pharmacology in drug development 8 (8), 1009–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk EM, Stadelmann AM, 2005. Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther. Drug Monit 27 (6), 799–810. [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Mental Health, 2013. Social Anxiety Disorder: Recognition, Assessment and Treatment. British Psychological Society [PubMed]
- Nazario LR, Antonioli R Jr., Capiotti KM, Hallak JE, Zuardi AW, Crippa JA, Bonan CD, da Silva RS, 2015. Caffeine protects against memory loss induced by high and non-anxiolytic dose of cannabidiol in adult zebrafish (Danio rerio). Pharmacol. Biochem. Behav. 135, 210–216. [DOI] [PubMed] [Google Scholar]
- Nordahl HM, Vogel PA, Morken G, Stiles TC, Sandvik P, Wells A, 2016. Paroxetine, cognitive therapy or their combination in the treatment of social anxiety disorder with and without avoidant personality disorder: a randomized clinical trial. Psychother. Psychosom 85 (6), 346–356. [DOI] [PubMed] [Google Scholar]
- O’Neill A, Wilson R, Blest-Hopley G, Annibale L, Colizzi M, Brammer M, Giampietro V, Bhattacharyya S, 2021. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol. Med 51 (4), 596–606. [DOI] [PubMed] [Google Scholar]
- Olfson M, King M, Schoenbaum M, 2015. Benzodiazepine use in the United States. JAMA Psychiatr. 72 (2), 136–142. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Yoshimura S, Yamawaki S, Yamaguchi S, Ura M, 2010. Does low self-esteem enhance social pain? The relationship between trait self-esteem and anterior cingulate cortex activation induced by ostracism. Soc. Cognit. Affect Neurosci 5 (4), 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME, 2006. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol. Psychiatr 59 (5), 424–429. [DOI] [PubMed] [Google Scholar]
- Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL, 2013. Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress. Anxiety 30 (3), 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachman S, Gruter-Andrew J, Shafran R, 2000. Post-event processing in social anxiety. Behav. Res. Ther 38 (6), 611–617. [DOI] [PubMed] [Google Scholar]
- Ray RD, Zald DH, 2012. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci. Biobehav. Rev 36 (1), 479–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey JA, Ghane M, Valdespino A, Coffman MC, Strege MV, White SW, Ollendick TH, 2016. Spatiotemporal dissociation of brain activity underlying threat and reward in social anxiety disorder. Soc. Cognit. Affect Neurosci 12 (1), 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodebaugh TL, Holaway RM, Heimberg RG, 2004. The treatment of social anxiety disorder. Clin. Psychol. Rev 24 (7), 883–908. [DOI] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK, 2005. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res 30 (8), 1037–1043. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ, 2007. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol 152 (7), 1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen J, Campbell DW, Leslie WD, Malisza KL, Stein MB, Paulus MP, Kravetsky LB, Kjernisted KD, Walker JR, Reiss JP, 2007. Striatal function in generalized social phobia: a functional magnetic resonance imaging study. Biol. Psychiatr 61 (3), 396–404. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Abi-Dargham A, Martinez D, Slifstein M, Hwang DR, Liebowitz MR, Laruelle M, 2009. Dopamine transporters, D2 receptors, and dopamine release in generalized social anxiety disorder. Depress. Anxiety 26 (5), 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG, 2002. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch. Gen. Psychiatr 59 (11), 1027–1034. [DOI] [PubMed] [Google Scholar]
- Stein MB, Walker JR, Forde DR, 1994. Setting diagnostic thresholds for social phobia: considerations from a community survey of social anxiety. Am. J. Psychiatr 151 (3), 408–412. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT, 2019. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. [DOI] [PubMed] [Google Scholar]
- Stewart SA, 2005. The effects of benzodiazepines on cognition. J. Clin. Psychiatr 66 (Suppl. 2), 9–13. [PubMed] [Google Scholar]
- Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH, 2004. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biol. Psychiatr 56 (12), 921–930. [DOI] [PubMed] [Google Scholar]
- Syal S, Hattingh CJ, Fouche JP, Spottiswoode B, Carey PD, Lochner C, Stein DJ, 2012. Grey matter abnormalities in social anxiety disorder: a pilot study. Metab. Brain Dis 27 (3), 299–309. [DOI] [PubMed] [Google Scholar]
- Taylor L, Gidal B, Blakey G, Tayo B, Morrison G, 2018. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs 32 (11), 1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa Y, Shibata M, Moriguchi Y, Umeda S, 2013. Anterior insular cortex mediates bodily sensibility and social anxiety. Soc. Cognit. Affect Neurosci 8 (3), 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG, 2007. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol 150 (5), 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillfors M, Furmark T, Marteinsdottir I, Fredrikson M, 2002. Cerebral blood flow during anticipation of public speaking in social phobia: a PET study. Biol. Psychiatr 52 (11), 1113–1119. [DOI] [PubMed] [Google Scholar]
- Tori ME, Larochelle MR, Naimi TS, 2020. Alcohol or benzodiazepine Co-involvement with opioid overdose deaths in the United States, 1999–2017. JAMA Netw. Open 3 (4), e202361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth A, Boczán J, Kedei N, Lizanecz E, Bagi Z, Papp Z, Édes I, Csiba L, Blumberg PM, 2005. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Mol. Brain Res 135 (1–2), 162–168. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhornitsky S, Li CS, Le TM, Joormann J, Li CR, 2019. Social anxiety, posterior insula activation, and autonomic response during self-initiated action in a Cyberball game. J. Affect. Disord 255, 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winton-Brown TT, Allen P, Bhattacharyya S, Borgwardt SJ, Fusar-Poli P, Crippa JA, Seal ML, Martin-Santos R, Ffytche D, Zuardi AW, Atakan Z, McGuire PK, 2011. Modulation of auditory and visual processing by delta-9-tetrahydrocannabinol and cannabidiol: an FMRI study. Neuropsychopharmacology 36 (7), 1340–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, Cosme RA, Graeff FG, Guimaraes FS, 1993. Effects of ipsapirone and cannabidiol on human experimental anxiety. J. Psychopharmacol 7 (1 Suppl. l), 82–88. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Rodrigues NP, Silva AL, Bernardo SA, Hallak JEC, Guimaraes FS, Crippa JAS, 2017. Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front. Pharmacol 8, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker I, Prendergast BJ, 2020. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex Differ 11, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


