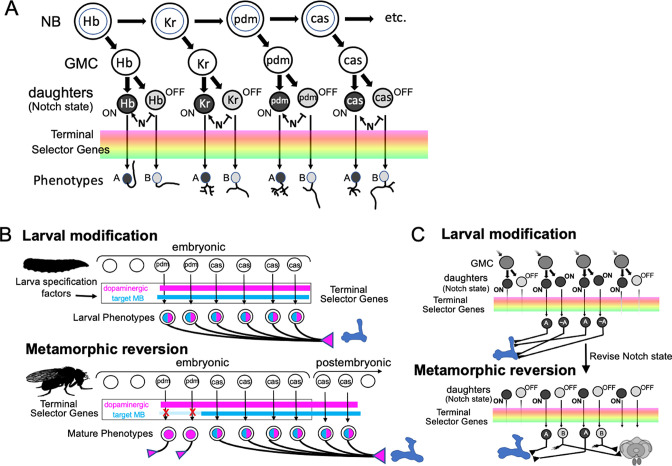
Figure 12. The origin of spatial-temporal information used to determine neuronal phenotypes.
(A) The early neuronal phenotypes within a lineage are determined by birth order of the ganglion mother cells (GMC). Birth order is encoded by a temporal program of transcription factor expression in the parent neuroblast (NB) as it divides. Transcription factor expression at the time of division is inherited by the GMC and its daughter neurons. Differences between the daughters is established by Notch (N) signaling with one sibling expressing the Notch-on (‘A’) fate and the other the Notch-off (‘B’) fate. This information, along with lineage identity factors, acts through a battery of terminal selector genes to establish neuronal phenotypes. (B) A hypothetical scheme to explain the metamorphic pattern of mushroom body input neuron (MBIN) recruitment and loss in the PPL1 cluster of dopamine neurons. It proposes that the Castor (cas) expressing neurons that are born in the DL1 lineage just before and after the embryonic neurogenic arrest are fated to become MBINs. The earlier born Pdm expressing neurons are also dopaminergic but their adult function is outside of the MB. Larval specification factors, though, modify how they interact with the terminal selector genes thereby transforming them into MBINs while the larval stage is maintained. (C) A hypothetical scheme using neurons of the DAL-V2/3 lineage to illustrate how sibling fates might be temporarily altered to recruit larval MBONs. In this scheme, two successive GMCs divide to produce one daughter that is an MBON and one that innervates the central complex, a dichotomy established by Notch signaling. With the evolution of the larva, Notch signaling is suppressed in the daughters during embryogenesis, allowing both to assume a similar fate – that of a larval MBON. With the reestablishment of normal Notch signaling at metamorphosis, the transformed daughter loses her MBON features and becomes a central complex neuron.