Figure 1: Study Consort.
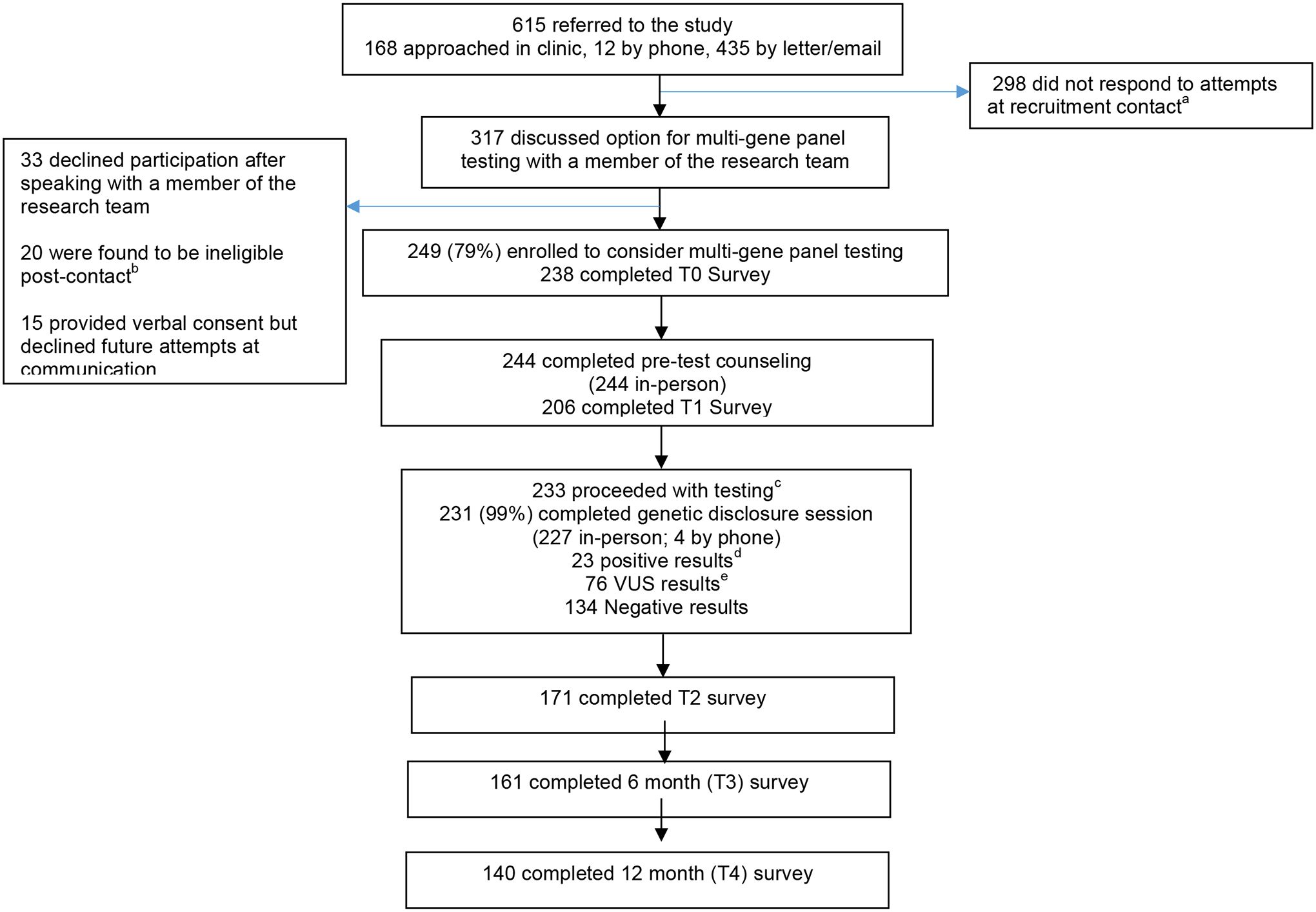
a Includes individuals who provided permission to be contacted during a clinic visit but never responded to recruitment attempts
b Ineligibility due to: offered a competing study (7), already had panel testing (6), previously tested positive for a genetic mutation (3), did not have prior BRCA1/2 testing (2), deceased (1), clinician request (1)
c three participants proceeded with a targeted custom panels.
d APC (9), ATM (4), BRCA1 (2), BARD1, CDH1, CHEK2 , APC/CHEK2, MUTYH (het), NBN, PALB2, TP53
e ATM (18), APC (7), CHEK2 (6), APC/CHEK2 (3), BRIP1 (3), RAD51D (3), SMAD4 (3), ATM/NBN (2), BMPR1A (2), BRCA1 (2), CHD1 (2), MSH6 (2), NBN (2), PMS2 (2), RAD51C (2), APC/CDH1, ATM/PMS2, ATM/BMPR1A, ATM/TP53, BARD1, BARD1/MSH2, BRCA1/MUTYH, BRCA2, BRIP1/CDKN2A, BRIP1/CHEK2, CDK4/CHEK2, CDKN2A, CDKN2A/TP53, CHEK2/STK11, MLH1, MUTYH/RAD51D, STK11.
