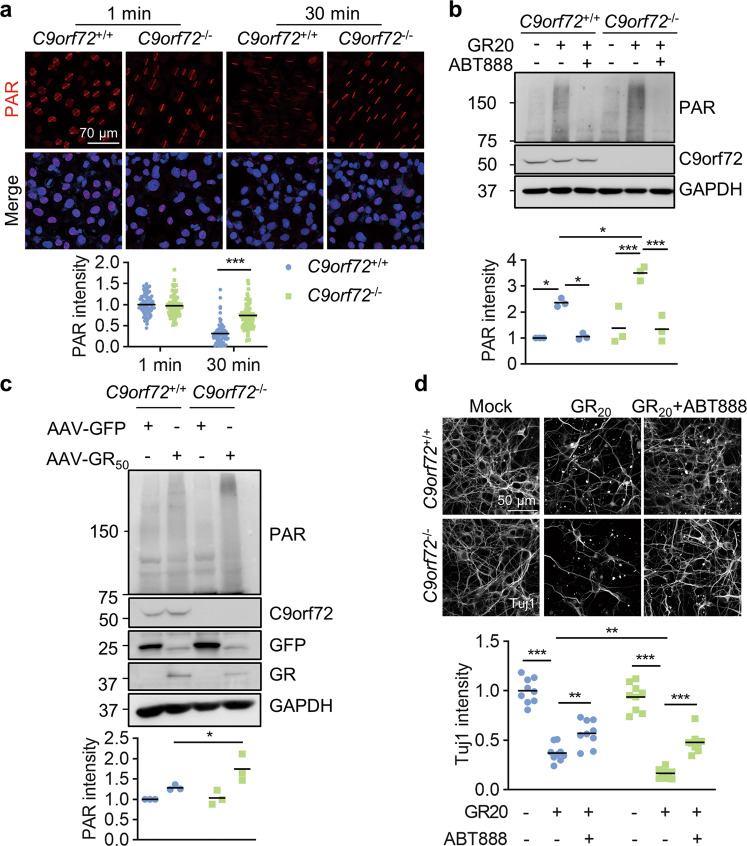
Fig. 8. C9orf72 deficiency exacerbates poly-GR-induced neuronal death by PARP-1 overactivation.
a Accumulated PAR polymers at laser-lesioned regions in C9orf72 KO cells. WT and C9orf72 KO U2OS cells were subjected to laser-generated DNA lesion by a 405 nm laser. The cells were fixed at 1 min or 30 min after microirradiation, and stained with anti-PAR antibody (red). Quantitative analysis of PAR intensity. Data are mean (n = 87–99 cells from 3 independent experiments; unpaired t-test; ***, p < 0.001). b Increased PAR in C9orf72 KO neurons after GR20 treatment. Primary neurons were cultured for 24 h and treated with poly-GR peptide (10 μM), with or without 10 μM ABT888 for 24 h. Proteins were analyzed by immunoblot. Data are mean (n = 3 independent experiments; Two-way ANOVA; *, p < 0.05; ***, p < 0.001). c Increased PAR polymers in the cortex of C9orf72−/−, AAV-GR50 mice. Proteins were extracted from the cortex of indicated genotypes and PAR polymers were analyzed by immunoblot. Quantitative analysis of PAR intensity. Data are mean (n = 3 independent experiments; paired t-test; *, p < 0.05). d PARP inhibition rescues poly-GR-induced neurodegeneration. The cortical neurons were cultured for 48 h and treated with poly-GR peptide (10 μM), with or without 10 μM ABT888 for 48 h. The neurons were fixed and stained with anti-Tuj1 antibody to reveal neurites. Quantitative analysis of Tuj1 intensity. Data are mean (n = 3 independent experiments; Two-way ANOVA; **, p < 0.01; ***, p < 0.001).