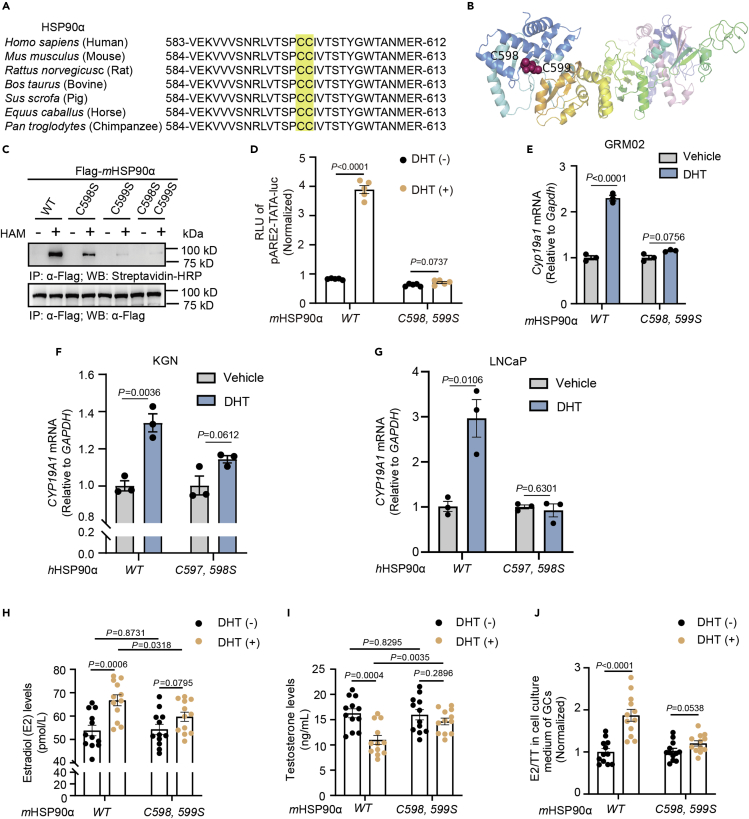
Figure 4.
HSP90α S-palmitoylation deficiency impairs DHT-induced AR activation
(A) Alignment of HSP90α protein sequences among different species.
(B) Ribbon representation of the S-palmitoylation sites on a mHSP90α 3D structure model. (Single-letter abbreviations for the amino acid residue: C, cysteine).
(C) Palmitoylation levels of Flag-tagged wild-type (WT) mHSP90α or mHSP90α (C598S), mHSP90α (C599S) and mHSP90α (C598S, C599S).
(D) DHT-induced transcriptional activation of a classic ARE-luciferase reporter in HEK293T cells (n = 5) transiently transfected with Flag-mHSP90αWT or S-palmitoylation-deficient Flag-mHSP90αC598, 599S. The data are presented as the means ± SEMs, and p values were determined by unpaired two-tailed Student’s t tests of n = 5 independent biological experiments.
(E) The GRM02 cells were transfected with Flag-mHSP90αWT or Flag-mHSP90αC598, 599S and then treated with DHT (100 nM) for 24h. Cell lysates were collected to determine the mRNA level of Cyp19a1.
(F and G) The KGN and LNCaP cells were transfected with Flag-hHSP90αWT or Flag-hHSP90αC597, 598S and then treated with DHT (10 nM) for 24h. Cell lysates were collected to determine the mRNA level of CYP19A1. Estradiol (H) and testosterone (I) levels in the supernatants of GRM02 cells (n = 12) transiently transfected with Flag-WT mHSP90α or S-palmitoylation-deficient Flag-mHSP90αC598, 599S after stimulation with DHT (100 nM) for 24h, as measured by ELISA.
(J) Estradiol to testosterone ratios, using the data from (H) and (I) (n = 12). In (D–F), all error bars, mean values ±SEM, and p values were calculated using unpaired two-tailed Student’s t tests.